Hydrocarbon
Hydrocarbons are organic compounds made up of only carbon and hydrogen atoms. Hydrocarbons are examples of group 14 hydrides. Because carbon has 4 electrons in its outermost shell (and because each covalent bond requires a donation of 1 electron, per atom, for bond formation) therefore carbon has exactly four bonds to make, and it is only established if 4 of these bonds are used. Aromatic hydrocarbons (arenes), alkanes, cycloalkanes and alkyl are compounds based on different types of hydrocarbons.
Chains of carbon atoms can be linear or branched, and open or closed. Those that have other chemical elements (heteroatoms) in their molecule are called substituted hydrocarbons.
Most of the hydrocarbons found on Earth occur naturally in crude oil, where decomposed organic matter provides an abundance of carbon and hydrogen that, when bound together, can catenate to form seemingly limitless chains.
Classification
Hydrocarbons can be classified into two types: aliphatic and aromatic. Aliphatics can be further classified into alkanes, alkenes, and alkynes based on the types of bonds that join carbon atoms together. The general formulas for alkanes, alkenes, and alkynes are CnH2n+2, CnH2n and CnH2n-2, respectively.
Saturated hydrocarbons or alkanes: they are compounds formed by carbon and hydrogen, they have single bonds (SP3). It has a general formula (CnH2n+2), where n is the number of carbons of the compound and the suffix -o and its ending in -ane.
CH4→ Methane, C2H6→Ethane, C3H8→Propane, C4H10→Butane, C5H12→Pentane, C6H14→ Hexane, C7H16→Heptane C8H18→Octane, C9H20→Nonane, C10H22→Decane.
According to the type of structures they can form, hydrocarbons can be classified as:
- Cyclical hydrocarbonswhich present their chains open. In turn, they are classified as:
- Linear hydrocarbons to those who lack lateral chains
- Branched hydrocarbonswhich have lateral chains.
- Cyclical hydrocarbons or Cycloalcanswhich are defined as closed chain hydrocarbons. These in turn are classified as:
- MonocyclicsThey have a single operation. Cycling.
- Polycyclicalcontaining several operations Cycling.
Polycyclic systems can be classified by their complexity into:
- Fusionwhen at least two cycles share a covalent link.
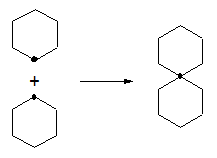
- Spiroalcanswhen at least two cycles have a single carbon in common.
- Bridges Structures of von BaeyerWhen a side chain of a cycle connects to any carbon. If it were connected to the cycle's carbon bonding with the chain, a spiro compound would be made. If the connection was on the cycle's neighboring carbon with the chain, it would have a merged compound. A connection in another carbon other than the previous ones generates a bridge.
- Groupswhen two independent cycles are connected through a covalent link.
- Cyclophanswhen from a cycle two chains connect to another cycle.
Based on the bonds between carbon atoms, hydrocarbons are classified as:
- Alphatic hydrocarbonswhich lack an aromatic ring, which in turn are classified as:
- Saturated hydrocarbons (alcans or paraffins), in which all their carbons have four simple links (or more technically, with sp hybridization)3).
- Unsaturated or unsaturated hydrocarbons, which have at least a double link (alquenos or olefinas) or triple (alkyno or acetylénic) in their carbon links.
- aromatic hydrocarbons, which present at least a structure that meets the Hückel rule (cyclical structure, that all of its carbons are of hybridization sp2 and that the number of electrons in resonance is not divisible between 4).
Hydrocarbons extracted directly from geological formations in a liquid state are commonly known as petroleum, while those found in a gaseous state are known as natural gas.
The commercial exploitation of hydrocarbons constitutes an economic activity of prime importance, since they form part of the main fossil fuels (oil and natural gas), as well as all kinds of plastics, waxes, and lubricants.
According to API degrees, they are classified as:
API=141,5GE− − 131,5where,GE=ρ ρ liquidρ ρ water{displaystyle API={frac {141,5}{GE}}}-131,5qquad {mbox{where, }qquad GE={frac {rho _{mbox{ liquid}}}}{rho _{mbox{ water}}}}}}}}{
If it is:
- ▪ 40 - condensed
- 30-39,9 - light.
- 22-29.9 - medium
- 10-21.9 - heavy.
- 9.9 - extra weight
Simple hydrocarbons and their variations
| Number of carbon atoms | Alcano (unique link) | (double link) | Rent (triple link) | Cycloalcan | Aleno |
|---|---|---|---|---|---|
| 1 | Methane | - | - | - | - |
| 2 | Etano | Etheno (ethylene) | Etino (acetylene) | - | - |
| 3 | Propane | Propene (propylene) | Propino (methylacetylene) | Cyclopropane | Propadieno (alleno) |
| 4 | Butano | Buteno (butylene) | Butino | Cyclobutan | Butadieno |
| 5 | Pentano | Penteno | Pentino | Cyclopentan | Pentadieno (piperilen) |
| 6 | Hexano | Hexeno | Hexino | Cyclohexane | Hexadieno |
| 7 | Heptano | Hepteno | Heptino | Cyclohepethane | Heptadieno |
| 8 | Octan | Octene | Octino | Cycloocethane | Octadie |
| 9 | Nonano | None | Nonino | Cyclone | No. |
| 10 | Dean | Decene | Decino | Cyclodean | Decadieno |
| 11 | A dean | Undecided | Undecino | Cycloundean | Undecadieno |
| 12 | Dodean | Dodecene | Dodecino | Cyclodean | Dodecadieno |
General properties
Due to differences in molecular structure, the empirical formula remains different between hydrocarbons; in linear or "straight-chain" alkanes, alkanes, and alkenes, the amount of hydrogen bonding decreases in alkenes and alkynes due to "self-bonding"; or carbon catenation that prevents the complete saturation of the hydrocarbon through the formation of double or triple bonds.
This inherent ability of hydrocarbons to bond to themselves is known as catenation, and it allows hydrocarbons to form more complex molecules, such as cyclohexane, and in rarer cases, arenes such as benzene. This ability comes from the fact that the bonding character between the carbon atoms is entirely nonpolar, in the sense that the distribution of electrons between the two elements is due in some way to the same electronegativity values of the elements (~0.30), and does not result in the formation of an electrophile.
Generally, with catenation comes the loss of the total amount of bonded hydrocarbons and an increase in the amount of energy required for bond cleavage due to stress exerted on the molecule; in molecules such as cyclohexane, this is known as ring strain, and occurs due to the "destabilized" of the atom.
In simple chemistry, according to valence bond theory, the carbon atom must follow the 4-hydrogen rule, which states that the maximum number of atoms available to bond with carbon is equal to the number of electrons that are attracted to the outer layer of carbon. In terms of shells, carbon consists of an incomplete outer shell, which comprises 4 electrons, and therefore has 4 electrons available for covalent bonding or dative bonding.
Hydrophobic hydrocarbons are like lipids.
Some hydrocarbons are also abundant in the solar system. Lakes of methane and liquid ethane have been found on Saturn's largest moon Titan, confirmed by the Cassini-Huygens mission.
Hydrocarbons are also abundant in nebulae that form polycyclic aromatic hydrocarbon (PAH).
Use
Hydrocarbons are a primary energy source for today's civilizations. The predominant use of hydrocarbons is as a fuel source. In their solid form, hydrocarbons take the form of asphalt (bitumen).
Volatile hydrocarbon mixtures are now used in preference to chlorofluorocarbons as a propellant for aerosols, due to chlorofluorocarbons' impact on the ozone layer.
Methane (CH4) and ethane (C2H6) are gases at room temperature and cannot be easily liquidated by pressure alone. Propane (C3H8) is, however, easy to liquefy, and exists in 'propane bottles' primarily as a liquid. Ethane and propane are promising feedstocks for the synthesis of ethylene and propylene. These alkenes are platform chemicals that allow the synthesis of derivatives (such as epoxide, ethanol, ethylene glycols, acetic acid, acrylic, acrylonitrile) and polymers (polyethylene, polypropylene, etc.). Butane (C4H10) is so easy to liquefy that it provides a safe and volatile fuel for small lighter bags. Pentane (C5H12) is a colorless liquid at room temperature, commonly used in chemistry and industry as a powerful, nearly odorless solvent for waxes and heavy weight organic compounds. molecular, including fats. Hexane (C6H14) is also a widely used non-aromatic and non-polar solvent, as well as a significant fraction of common gasoline. The C6 through C10 alkanes, isomeric alkanes and cycloalkanes are the major components of blends of gasoline, naphtha, jet fuel and specialty industrial solvents. With the progressive addition of carbon units, simple ring-structured hydrocarbons have higher viscosities, lubrication indices, boiling points, solidification temperatures, and deeper color. At the opposite end of methane are heavy tars which remain as the lower fraction in a refining replica of crude oil. They are widely collected and used as roofing compounds, flooring composition, wood preservatives (the creosote series), and as extremely high viscosity shear strength liquids.
The use of hydrocarbons is also frequent in nature. Some eusocial arthropods, such as the Brazilian stingless bee, Schwarziana quadripunctata, use "odors" hydrocarbons to determine the relationship between non-relatives. The chemical composition of hydrocarbons varies between age, sex, nest location and hierarchical position.
Toxicity
Poisoning by hydrocarbons such as benzene and petroleum often result accidentally from inhaling or ingesting these cytotoxic chemicals. Intravenous or subcutaneous injection of petroleum compounds with the intent of suicide or abuse is an extraordinary event that can result in local damage or systemic toxicity such as tissue necrosis, abscess formation, respiratory system failure, and partial damage to the kidneys, brain, and the nervious system. Moaddab and Eskandarlou report a case of chest wall necrosis and empyema resulting from a suicide attempt by injection of petroleum into the pleural cavity.
Reactions
There are three main types of hydrocarbon reactions:
- Replacement reaction
- Addendum reaction
- Combustion
Substitution Reactions
Substitution reactions only occur in saturated hydrocarbons (carbon-carbon single bonds). In this reaction, an alkane reacts with a chlorine molecule. One of the chlorine atoms displaces a hydrogen atom. This forms hydrochloric acid as well as the hydrocarbon with a chlorine atom.
- CH4 + Cl2 → CH3Cl + HCl
- CH3Cl + Cl2 → CH2Cl2 + HCl
up to CCl4 (carbon tetrachloride)
- C2H6 + Cl2 → C2H5Cl + HCl
- C2H4Cl2 + Cl2 → C2H3Cl3 + HCl
up to C2Cl6 (hexachloroethane)
Addition reactions
Addition reactions involve alkenes and alkynes. In this reaction a halogen molecule breaks the double or triple bond in the hydrocarbon and forms a bond.
Combustion
Hydrocarbons are currently the main source of electrical energy and heat (such as heating homes) due to the energy produced when burned.
Often this energy is used directly as heat, such as in home heaters, which use either oil or natural gas. The hydrocarbon is burned and the heat is used to heat the water, which is then circulated. A similar principle is used to create electrical energy in power plants.
Common properties of hydrocarbons are the fact that they produce steam, carbon dioxide and heat during combustion and oxygen is necessary for combustion to occur. The simplest hydrocarbon, methane, burns as follows:
- CH4 + 2 O2 → 2 H2O + CO2 + energy
In an inadequate supply of air, carbon monoxide gas and water vapor are formed:
- 2 CH4 + 3 O2 → 2 CO + 4 H2O
Another example is the combustion of propane:
- C3H8 + 5 O2 → 4 H2O + 3 CO2 + energy
And finally, for any liner alkane of n carbon atoms,
- CnH2n+2 + 3n + 1/2O2 → (n+ 1) H2O + nCO2 + energy
The burning of hydrocarbons is an example of an exothermic chemical reaction.
Hydrocarbons can also be burned with elemental fluorine, resulting in products carbon tetrafluoride and hydrogen fluoride.
Oil
Hydrocarbons extracted in liquid form are called petroleum (literally "rock oil") or mineral oil, while hydrocarbons in gaseous form are called natural gas. Oil and natural gas are found underground with the tools of petroleum geology and are an important source of fuel and raw materials for the production of organic chemicals.
Liquid hydrocarbon fuel extraction from sedimentary basins is an integral part of modern energy development. Hydrocarbons are mined from tar sands and oil shale, and potentially extracted from sedimentary methane hydrate. These reserves require distillation and upgrading to produce synthetic crude and oil.
Oil reserves in sedimentary rocks] are the source of hydrocarbons for the energy, transportation, and petrochemical industries.
Hydrocarbons of economic importance include fossil fuels such as coal, oil, and natural gas, and their derivatives such as plastics, paraffins, waxes, solvents, and oils. Hydrocarbons – along with NOx and sunlight. – contributes to the formation of tropospheric ozone and greenhouse gases.
Bioremediation
Bacteria in the gabbroic layer of the ocean crust can break down hydrocarbons; but the extreme environment makes research difficult. Other bacteria such as Lutibacterium anuloederans can also degrade hydrocarbons. Mycoremediation or breakdown of hydrocarbons by mycelium and fungi is possible.
Security
Many hydrocarbons are highly flammable; therefore, care must be taken to avoid injury. Benzene and many aromatic hydrocarbons] are possible carcinogens, and proper safety equipment must be worn to prevent these harmful compounds from entering the body. If hydrocarbons burn in tight areas, toxic carbon monoxide can form. Hydrocarbons must be kept away from fluorine compounds due to the high probability of formation of toxic compounds such as hydrofluoric acid.
Environmental impact
Hydrocarbons enter the environment through their extensive use as fuels and chemicals, as well as through accidental leaks or spills during exploration, production, refining, or transportation. Anthropogenic soil contamination by hydrocarbons is a serious global problem due to the persistence of pollutants and the negative impact on human health.
Substituted hydrocarbons
Substituted hydrocarbons are compounds that have the same structure as a hydrocarbon, but contain atoms of elements other than hydrogen and carbon instead of a part of the hydrocarbon. The part of the molecule that has a specific arrangement of atoms, which is responsible for the chemical behavior of the base molecule, is called the functional group.
For example:
The halogenated compounds have halogen atoms as their functional group. They have a high density. They are used in refrigerants, solvents, pesticides, moth repellents, in some plastics, and in biological functions: thyroid hormones. For example: chloroform, dichloromethane, thyroxine, Freon, DDT, PCBs, PVC. The structure of halogenated compounds is: R-X, where X is fluorine (F), chlorine (Cl), bromine (Br) and iodine (I), and R is a hydrocarbon radical.
Microorganisms that break down hydrocarbons
Microorganisms are considered as beings capable of adapting and adapting their metabolism depending on the environmental conditions in which they develop and the physical-chemical parameters that they present, which also allows them to develop in places where hydrocarbons are present.
There are around 160 genera of microorganisms that degrade hydrocarbons, among the main ones are:
- Acinetobacter
- Flavobacterium
- Corynebacterium
- Bacillus
- Achromobacter
- Rhodococcus
Contenido relacionado
Coordination link
Potassium nitrate
Boron