Heat engine
A thermal engine is a thermal machine that transforms heat into mechanical work by taking advantage of the temperature gradient between a heat source (hot source) and a heat sink (cold source).. Heat is transferred from the source to the sink, and during this process, some of the heat is converted to work by taking advantage of the properties of a working fluid, usually a gas or the vapor of a liquid.
The heat required for the proper functioning of a heat engine comes from the chemical energy released in combustion, being absorbed by a motor fluid that sets a series of mechanical parts in motion.
If the combustion takes place outside the engine, the engines are called external combustion engines, and if the combustion takes place inside the engine, the engines are called internal combustion engines. The movement produced can be reciprocating or rotating.
The motor fluid is usually water vapor, air or a mixture of gases resulting from the combustion of oil or fuel gases. In internal combustion engines, combustion takes place in the motor fluid, and in external combustion engines there are two fluids, where heat is exchanged between them. In nuclear power plants, the heat comes from the energy released in the nuclear fission of uranium or plutonium, being extracted by a refrigerant substance that transfers it to a secondary circuit through a heat exchanger.
History
In 1765 the Scotsman James Watt manufactured the first efficient steam engine, and it also marked the beginning of the Industrial Revolution, born as a consequence of the replacement of muscular effort by the work of machines. The steam engine was a triumph of technology that gave rise to factories in most countries, while raising the level of welfare of society.
A century later, internal combustion engines revolutionized land, sea, and air transportation. Science gave rise to the International System of Units.
Steam engine
The steam engine is an external combustion engine that uses the expansive force of water vapor to move a piston and produce work.
History of the steam engine
In 1691, English military engineer Thomas Savery pioneered the use of steam pressure to draw water from mines and wells to drive a waterwheel. Normally the pressure of the water vapor used to burst boilers and pipes, and also, it was ineffective because the heat of the water vapor was lost every time the container cooled.
In 1698 Thomas Savery patented his discovery, and the English blacksmith Thomas Newcomen built a more perfect machine, which worked at low pressures. In addition, it had a piston and a cylinder. With air pressure he could move the piston.
In 1765 Scottish mechanic James Watt improved on Thomas Newcomen's engine, and in 1782 he built the first steam engine.
In 1787 American inventor John Fitch built a steamboat, but it failed financially.
In 1807 Robert Fulton launched the Clermont, the first steamship.
In 1814 the Englishman George Stephenson built the first steam locomotive.
How the steam engine works
- Home: Exterior of the machine, where the combustion is performed.
- Caldera: Recipient of steel where water vapor is generated. It is equipped with a level of water to observe the height of it, a gauge to measure the inner pressure, and a safety valve that opens when the pressure reaches a certain value, thus avoiding an explosion. The water is driven by a pump and penetrates into the boiler in liquid state, at high pressure and at room temperature. In the boiler the water heats and vaporizes to an overheater connected to the boiler, where the temperature increases and keeps the pressure. The water vapor finally passes to the cylinder.
- Cylinder engine: It is made up of the distribution box, the belt and the piston. The water vapor penetrates into the distribution box and enters into contact with the piston while the water vapor is pushed to an orifice where it escapes heading towards the fireplace or the capacitor. In the capacitor, the remaining steam is ducted and yielded to the refrigerant. The condensed liquid is sent back by the pump to the boiler, thus completing the cycle.
- Movement transforming bodies: These organs are the stem, the biela and the crank, three articulated rods that transform the alternative movement of the embolo into circular motion. The stem is attached to the biela through a cross. The crank turns to an inertia steering wheel, whose mission is to keep the angular speed constant. The steering wheel is fitted with an eccentric, which regulates the movement of the sliding.
Rankine Cycle
The process of expansion of the steam against the piston is adiabatic, but not reversible, so it is not isentropic.
For a heat engine to follow the Carnot cycle, it would be necessary to stop the condensation of the vapor before it is completely liquefied and, by means of a compressor, ensure that the vapor-liquid mixture reaches its complete liquefaction at the temperature of the boiler.
Since this proved impossible, the Scottish engineer J. M. Rankine proposed a modification of the Carnot cycle, called the Rankine cycle.
- Liquid water from the boiler absorbs the warmth of the home, elevates its temperature to constant pressure. Maintaining constant temperature and pressure is transformed into saturated and dry steam reversibly.
- The saturated steam expands inside the machine in adiabatic and reversible way, until it reaches the temperature of the refrigerator and is condensed. Work in this case is positive.
- Condensation continues at constant temperature and pressure, thus forming saturated liquid.
- The saturated fluid is compressed to the temperature and pressure of the boiler.
In a Rankine cycle with superheat, the average temperature at which heat is absorbed implies higher performance. The steam remains dry throughout the adiabatic expansion, thus reducing the dangers of corrosion.
Power of the steam engine
The power of a steam engine depends on the pressure and the amount of water vapor admitted by the cylinder in the unit of time. As the pressure varies, an average value called the mean effective pressure is considered.
The amount of water vapor per unit time is equal to the volume of the cylinder corresponding to each revolution multiplied by the number of revolutions in unit time. The volume is calculated by multiplying the piston section by the length of the stroke.
P=pLSf{displaystyle P=pLSf}being
- P{displaystyle P} the power of the machine,
- p{displaystyle p} effective average pressure,
- L{displaystyle L} the length of the race,
- S{displaystyle S} the section of the piston,
- f{displaystyle f} the number of revolutions per unit of time.
When the steam acts on both faces of the piston, the theoretical power developed is twice this value.
Because of friction and other losses, the actual power is usually 70 or 90% of the quoted power.
The size of the steam engine is limited to powers of 1000 CV, speeds of 213 m/min, pressures of 14 kp/cm², temperatures of 315 °C and efficiencies of 30%.
Steam engines have been used as engines for locomotives, and ships. Currently, they have been replaced by combustion engines.
Steam turbine
In the steam turbine, the water vapor is distributed through four tubes and acts directly on the blades of a wheel, making it rotate at a speed of about 10,000 rpm.
The steam turbine lacks a motor cylinder and transformer organs of movement, so the performance is higher.
Currently, the steam turbine is used in power plants, ship propulsion and blast furnace blowing installations.
Postulates of Thermodynamics
- Whatever the procedure used to convert heat to work or vice versa, there is a constant relationship between the work developed and the heat consumed, provided that the final state of the system is equal to the initial (thermodynamic cycle). The mechanical equivalent of heat is 427 kg/kcal or in the international standards system ISO 4184 July/1000 cal.
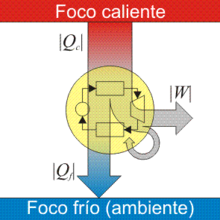
- A thermal machine can only perform work if it absorbs heat from a spring at higher temperature and yields it in part to another at lower temperature. That is, heat cannot spontaneously be transferred from a colder body to a warmer body.
Basic operating principle
In a heat engine, a series of transformations take place that lead to an initial state (that is, it has a closed cycle). In the course of these transformations, the engine receives thermal energy in the form of heat and returns mechanical energy in the form of work.
Efficiency of heat engines
Efficiency of various heat engines proposed or used today ranges from 3% (97% wasted heat) for ocean thermal energy conversion systems, to 25% for most automobile engines, 35% for a supercritical coal-fired power plant, and 60% for a steam-cooled combined-cycle gas turbine. All of these processes gain their efficiency (or lose it) due to the depression of temperature across them. For example, ocean thermal energy conversion systems use a temperature difference between the water on the surface and the water in the deep ocean, i.e. a difference of perhaps 25°C, so the efficiency should be be low Combined cycle turbines use natural gas burners to heat air to around 1,530°C, or up to 1,500°C difference, so efficiency can be higher when the steam cooling cycle is added.
Classification of heat engines
For the classification of heat engines, in addition to the criteria already mentioned in the case of fluid machines, two additional aspects are taken into consideration:
- If the fluid is capacitable (water) or non-condensable (air).
- If the process is external or internal combustion.
Internal combustion engines
The foundation of internal combustion engines is the realization of combustion inside the cylinder of the machine, in which the motor agent is the fuel mixed with the air necessary for combustion.
There are different types of internal combustion engines depending on the fuel used, the combustion conditions and the number of strokes the piston makes in one cycle. The movement can be reciprocating, which is carried out by the explosion and combustion engines, or rotary, which is carried out by the explosion and combustion turbines.
In reciprocating motion machines, combustion is instantaneous, produced by an electric spark, and gaseous fuels or highly volatile liquids must be used, such as gasoline. In rotary motion machines, it is carried out progressively and at constant pressure, using less volatile liquid fuels, such as diesel.
In internal combustion machines, the combustion gases are those that circulate through the machine itself. In this case, the machine will necessarily have an open cycle, and the motor fluid will be air (non-condensable) used as an oxidizer in combustion.
| Internal combustion engines |
|---|
| Rotative | Turbomachine | Open cycle gas turbine |
| Volumetric | Wankel Motor, Quasiturbina | |
| Alternative | Powered by compression | Diesel engine |
| Ignited | Explosion engine (Otto, Miller, poor mixer, Atkinson Cycle) | |
| Reaction | Motor rocket | Liquid/solid space cohete |
| Aeroreactor without compressor | Statorrector Pulsorreactor | |
| Aeroreactor with compressor | Turborreactor Turbofán Turbohélice |
Explosion or provoked ignition (MEP) engines
The most commonly used internal combustion engine is the four-stroke engine, which is made up of the following components.
- Inyector: Mechanical or electrical device for injecting gasoline into the cylinder or in the intake duct. The old explosion engines had a carburetor, where the gasoline was sprayed and mixed with the air.
- Cylinder: It is made up of the body of the pump, a piston, an admission valve and another exhaust valve, and a spark plug. In the cylinder the explosion of the fuel is performed, originating an alternative movement of piston or piston. The piston has an inverted form of glass and is attached to the biela by means of a bun, it must be resistant to mechanical and thermal efforts and is provided with segments hosted in slots at the top.
- The intake and exhaust valves allow the entry of the fuel and the exit of the combustion gases. They are located in the canopy, on the cylinder, and are kept in their closing position by a dock, opening towards the interior by means of a cam. The cams are located in the cam tree, which is synchronized with the crankshaft.
- The canopy is a piece that closes the cylinders in the combustion zone, and is coupled with bolts. The high temperatures reached in the bowl require a cooling system, which can be by air or by water. The air cooling system provides cooling fin cylinders, and the water cooling system circulates between the cylinder and canopy deck, where the water runs through the circuit driven by a pump and cools in the radiator through an air current produced by a fan.
- The spark plug is formed by two separate electrodes about 0.5 mm. One of them is attached to a mass, and another comes from the distributor, both electrically isolated. Bujía produces a spark that explodes the fuel.
- The intake and exhaust valves allow the entry of the fuel and the exit of the combustion gases. They are located in the canopy, on the cylinder, and are kept in their closing position by a dock, opening towards the interior by means of a cam. The cams are located in the cam tree, which is synchronized with the crankshaft.
- Movement transforming bodies: The transforming organs of the movement are the biela-manivela and the crankshaft, which transform the alternative movement of the piston into circular motion. The biela transmits the piston effort to the cigüeñal, and this transmits the power developed in the cylinders of the engine tree. The cylinders, the biela-manivela and the crankshaft are locked in the frame-carter, which must be resistant to the efforts of the piston, and also protects all the elements, and serves as a lubricating tank.
- It is necessary a lubrication due to the amount of mobile parts, not only to increase performance, but to avoid deterioration. The walls of the cylinder, the joints of the bielas, the cam tree, the valves, the crankshaft bearings and the gears must be particularly lubricated. Lubrication is carried out through a pressure oil circuit. The oil is in a carter, from which it is distributed through a pump.
Otto's Cycle
A heat engine follows the cycle devised in 1862 by Beau de Rochas and used for the first time in 1877 by Nikolaus Otto, thus calling the cycle the Otto cycle.
The Otto cycle is carried out by a perfect gas, and consists of two adiabatic processes and two isochores, which are called times.
- First time: Admission. Lowers the piston, opens the intake valve and enters by aspiration the fuel and air in the cylinder.
- Second time: Compression. Upload the piston, close the valves and compress the fuel.
- Third time: Explosion-expansion. When you reach the maximum compression you jump the spark of the spark plug, explode the fuel and throw the piston down. During this process the piston is located at the highest part, and the valves remain closed.
- Fourth time: Escape. The exhaust valve is opened and the piston drives out the gases.
The performance of the Otto cycle is given by the expression:
MIL MIL =1− − 1Rγ γ − − 1=1− − 1(V1V2)γ γ − − 1{displaystyle eta =1-{frac {1}{R^{gamma -1}=1-{frac {1}{({frac {V_{1}}}{V_{2}}}}}}})^{gamma -1}}}}}}}where R is the degree of compression of the mixture, and γ γ {displaystyle gamma } It's the adiabatic coefficient.
In gasoline engines there is a limit above which the degree of compression cannot be raised, since at high temperatures and pressures the fuel explodes before the spark occurs. It is said that the autoignition level has been reached.
This knock produces an audible shock that damages the engine and decreases performance. Adding anti-knock substances or catalysts, compression degrees of 8 to 10 are achieved.
The reasons for poor performance are as follows:
- Combustion is not usually complete, and carbon monoxide is produced.
- There is a heat exchange between gases and walls, which forces to cool the cylinder.
- The combustion is not instantly verified, and there is a volume increase. In order to correct it, an ignition progress is made, which is to cause the explosion before the piston has made the time.
Gasoline octane rating
Gasolines with high anti-knock power are those in which cyclic hydrocarbons and branched-chain hydrocarbons predominate, which detonate with greater difficulty. To compare the antiknock properties, the octane index or number is used, where isooctane and n-heptane are taken as hydrocarbons, to which antiknock powers of 100 and 0 are assigned.
The octane index or number is the percent by volume of isooctane in a mixture of isooctane and n-heptane that has the same anti-knock power as gasoline.
Aviation gasoline has an octane number greater than 100, and consists of 20% aromatic hydrocarbons.
In order to reduce detonation and increase the octane index or number of a gasoline, antiknock agents were used in the past, which acted as negative catalysts for the combustion reactions of hydrocarbons. The most complete was tetraethyl lead, which was added at 0.1% to gasoline. When the gasoline exploded, metallic lead and lead oxide were released, which damaged the engine and polluted the air. Currently, unleaded gasolines are used using other types of catalysts.
External combustion engines
The causes that limit the performance of external combustion machines lie in the impossibility of reaching high temperatures in the boiler, due to the pressure that is reached; and the difficulty of making use of the calorific energy of coal and other fuel.
If the combustion is external, the heat of combustion is transferred to the fluid through a wall, for example in a heat exchanger. This type of machine does not require a combustion process, as occurs in nuclear facilities, although it is the usual procedure. Since the motor fluid does not undergo any degradation, these machines can be closed-cycle, which is currently the trend for economic reasons.
| External combustion engines |
|---|
| Fluid condenser | Turbomachine | Open or closed steam turbine |
| Alternative | Steam machine open or closed | |
| Non-flux condenser | Turbomachine | Closed cycle gas turbine |
| Alternative | Stirling Motor |
- NOTE: Rotative and reaction volumetric motors have not been developed.
Contenido relacionado
Rutherford's atomic model
Air safety
Ether (physics)