Gunpowder
Gunpowder is an explosive mixture used mainly as a propellant for projectiles in firearms and for acoustic and visual purposes in fireworks. The word gunpowder refers specifically to the so-called black powder. It is made up of certain proportions of carbon, sulfur and potassium nitrate, but with the appearance of modern nitrocellulose propellants, this name was extended to these, despite being chemically distinct products.
The most popular gunpowder is 75% potassium nitrate, 15% carbon, and 10% sulfur (percentages by mass). It is currently used in pyrotechnics and as a projectile propellant in ancient weapons. Modern smokeless powders are based on energetic materials, mainly nitrocellulose (monobasic) and nitrocellulose plus nitroglycerin (bibasic). The advantages of modern gunpowders are their low level of smoke, low level of deposits of combustion products in the weapon and their homogeneity, which guarantees a consistent result, thereby increasing the accuracy of the shots.
History
The consensus among the different currents of study is that gunpowder was invented in China, distributed in the Middle East and introduced to Europe by the Middle East; however, there is no consensus on how this Chinese military invention influenced technological advances about gunpowder in the Middle East and Europe. The distribution of gunpowder across Asia from China is largely attributed to the Mongols. One of the earliest examples of Europeans facing off against armies with firearms was the Battle of Mohi, in 1241. In this battle the Mongols used gunpowder in both firearms and grenades.
Chinese
Gunpowder was discovered in China when Taoists were trying to create a potion for immortality. The Chinese military forces used weapons based on gunpowder (rockets, muskets, cannons) and explosives (grenades and different types of bombs) against the Mongols when they tried to enter their lands on the northern border. After the Mongols conquered China and founded the Yuan Dynasty, they used Chinese military technology for their attempted invasion of Japan, where they also used gunpowder to power their rockets.
Medicinal origin
Saltpeter was known to the Chinese since before the 1st century B.C. and there is clear evidence of the use of saltpeter and sulfur in many medical combinations. A Chinese alchemist text, dated 492 AD. C., mentions saltpeter burning with a purple flame, showing it as a practical and reliable way to distinguish it from other inorganic salts, thus allowing alchemists to evaluate and compare purification techniques. The oldest records of saltpeter purification date before 1200 AD. C.
The first reference to the incendiary properties of such mixtures is the passage from Zhenyuan miaodao yaolüe, a Taoist text dated to the mid-9th century AD. C.
Some have heated sulfur together, scold, and salute with honey; they get smoke and flames, burning their hands and their faces, and even the whole house where they were working burned.
The Chinese word for "gunpowder" is in Chinese, 火药/火藥; pinyin, huŏ yào /xuou yɑʊ/, which literally means "fire medicine". However, this name only came into use a few centuries after the discovery of the mixture. During the 9th century AD. C. Taoist monks or Chinese alchemists looking for the elixir of immortality accidentally found gunpowder.
Warfare use
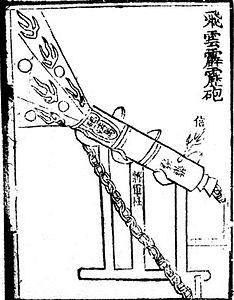
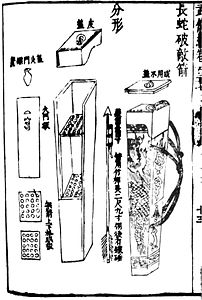
The Chinese were quick to use gunpowder for warfare and over the next few centuries produced a wide variety of firearms, including flamethrowers, rockets, bombs, and landmines before inventing the modern firearm, which uses a metallic projectile. Archaeological evidence of a hand cannon dated to the late XIII century was found in Manchuria and explosive bombs have been discovered in a shipwreck off the coast of Japan, dated to 1281, during the Mongol invasions of Japan.
The Chinese work "Wu Ching Tsung Yao" (Essential Fundamentals of Classical Militia), written by Tseng Kung-Liang between 1040-1044 AD. C., provides encyclopedic references to a variety of mixtures that included petrochemicals—as well as honey and garlic. A slow-acting fuse for flame-throwing mechanisms using the siphon principle for rockets and fireworks is also mentioned. However, the recipes for the mixtures in this book do not contain enough saltpeter to create an explosive, being limited to a maximum of 50% saltpeter they only produce an incendiary effect. This work was written by a court bureaucrat of the dynasty Song, and there is thought to be little evidence of this work's immediate impact on armed conflict. There is no mention of the use of gunpowder in the chronicles of the wars against the Tanguts in the XI century AD. C., in addition to the fact that China at this time was in a situation of peace. The first chronicle of the use of "fire lances" it is on the site of De'an in 1132.
Middle East
Muslims acquired knowledge of gunpowder between 1240-1280, when the Syrian Hasan al-Rammah had written in Arabic, recipes for gunpowder, instructions for saltpeter purification, and descriptions of incendiary weapons. Gunpowder reached the Middle East possibly via India and China. This can be deduced from the way al-Rammah used to call gunpowder, where he called saltpeter "Chinese snow" (in Arabic, ثلج الصين thalj al-ṣīn), to fireworks as "Chinese flowers" and rockets as "Chinese arrows". The Persians called nitrate "Chinese salt" or "salt of the Chinese salt marshes" (in Persian, نمک شوره چيني namak shūra chīnī).
Al-Hassan claimed at the Battle of Ain Jalut in 1260 that the Mamluks used in "the first cannon in history" against the Mongols a mixture of gunpowder with a composition nearly identical to explosive gunpowder. The earliest known surviving documentary evidence for the use of the hand cannon, considered the oldest type of firearm, comes from various Arabic manuscripts 14th century. Al-Hassan argues that these are based on older originals and that they report that the Mamluks used cannons at the battle of Ain Jalut in 1260. Hasan al-Rammah included 107 recipes for gunpowder in his work al-Furusiyyah wa al-Manasib al-Harbiyya (The Book of Chivalry military and ingenious devices of warfare) and 22 recipes for rockets, where these recipes had a composition almost identical to the modern composition of gunpowder.
Europe
Gunpowder was invented in China around the 9th century AD. The Byzantines and Arabs introduced it to Europe around 1200. It is likely that gunpowder was brought to Europe from the Near East. The first reference to its manufacture in Europe is found in a document by Roger Bacon, the Epistola de secretis operibus Artis et Naturae, et de nullitate Magiae (ca. 1250). In this text we read:
Accipiatur igitur de ossibus Adae, et de calce sub eodem pondere; et sint sex ad lapidem Tagi, et quinque ad lapidem unionis; et terantur simul cum aqua vitae, cujus proprium est dissolvere omnes res alias, ita quod in ea dissolvantur et assentur. Et iteretur multotiens contrition et assatio, doncin cerentur; hoc est ut uniantur parts, sicut in cera. Et signum incerationis est, quod medicina liquescit super ferrum valde ignitum; deinde ponatur in eadem aqua in loca warm and humido, aut supendatur in vapore aquarum valde calidarum; deinde dissolvantur, et congelentur ad solem. Dein accipies sal petrae, et argentums vivum convertes in plumbum, et iterum plumbum eo lavabis et mundificabis, ut sit próxima argento, et tunc operare ut prius. Item pondus totum sit 30. Sed tamen sal petrae LURU VOPO VIR CAN VTRIET sulphuris; et sic facies tonitruum et coruscationem, sias artificium - Cap. XI
Berthold Schwarz, a German monk, in the early XIV century, may have been the first to use gunpowder to drive a projectile, although it seems that at the same time the Arabs had already used it for the same purpose in the Iberian Peninsula, according to the chronicles of King Alfonso XI of Castile. The following paragraph, transcribed and adapted to modern Spanish, corresponds to the chronicle of King Alfonso XI on the siege of Algeciras (1343), and is the first written reference to the use of gunpowder for military purposes, although there are those who maintain that this The same substance had already been used, also by the Arabs, in the defense of the city of Niebla (Huelva) when it was besieged by Alfonso X the Wise, almost a century earlier.
..threw [the Arabs] many [bolas] of iron that cast them with thunder, of which Christians felt a great fright, since any member of the man that was reached was closed as if they cut him with a knife; and as he wanted the man to fall wounded he died afterwards, for there was no surgery that could cure him, on the one hand because they came [the pelles] burning like fire,..et they gave him with a thunder in his arm, and cutongelo, and then died another day: and that same thing happened to those who were fed of thunder. Et even the estoria is counting on the fechos de la hueste.
Whatever the precise data and the identities of its discoverers and first users, the truth is that gunpowder was manufactured in Italy in 1326, in England in 1334 and that in 1340, in territories that today belong to Germany, it was counted with facilities to produce it. The first attempt to use gunpowder to undermine the walls of fortifications takes place during the siege of Pisa (Italy) in 1403. In the second half of the century XVI, the manufacture of gunpowder was a state monopoly in most countries. It was the only known explosive until the discovery of so-called percussion gold, a powerful explosive used for the first time in 1628 during the warfare that took place on the European continent.
Indian
Gunpowder and firearms were brought to India through the Mongol invasions of India. Ottoman admiral Seydi Ali Reis introduced the first versions of matchlock firearms, which the Ottomans used against the Portuguese at the Siege of Diu (1531). After that many varieties of firearms were introduced in Tanjore, Dhaka, Bijaour and Murshidabad.
The Mughal Empire mass-produced matchlock firearms for its army. The Mughal Empire was the first to develop bamboo rockets, mainly for signaling and for use by sappers. The Mughal emperor clashed with the British and other Europeans in the province of Gujarat, where Europeans extracted saltpeter for their gunpowder during the 17th century XVII.
In 1780 the British began to annex the territories of the Mysore Sultanate, during the Second Anglo-Mysore War. The British battalion was defeated during the Battle of Guntur, by the forces of Hyder Ali, who effectively used Mysore rockets and rocket artillery against the tightly ranked British troops. This technology was copied and used in the Napoleonic wars in Europe.
Indonesia
Documentary and archaeological evidence indicates that gunpowder, muskets and cannons were introduced to Indonesia by Arab or Indian traders around the 14th century The Portuguese and Spanish invaders met with these firearms and were generally outmatched. The Singhasari empire had firearms and cannons. The Javanese had bronze cannons, used extensively by the Majapahit army as well as by pirates.
America
Gunpowder was introduced to America by the Spanish and Portuguese conquerors who used it against the Aztecs, Mayans, Incas, etc. Saltpeter and sulfur deposits could be easily found in various regions of Mexico, so the forces of the conquistadores were able to replenish the gunpowder used by their firearms.
And for the munition no less God provided, that we found so much salitre and so good that we could provide for other needs, having pairs of boilers to cook it, although it is spent here fed up in the many entrances that are made. And for the sulfur already to Your Sacra Majesty, I have made mention of a saw that is in this provience that comes out a lot of smoke, and from there, entering a Spanish seventy or eighty braces tied to the bottom has been pulled, with which until agora we have held.Hernan Cortes, Relationship letters
It is popularly believed that firearms were a determining factor in the defeat of local civilizations, however archeological and documentary evidence shows that firearms carried by Europeans were not yet as effective and had little tactical advantage, they also did not cause panic in the local inhabitants as is popularly believed, since the local forces quickly got used to their use. They even learned how to work muskets and cannons, avoiding being hit by them.
The Spaniards who were going in the bergantines turned them, the artillery where the canoes were thicker...Seeing this, the Mexicans began to depart and keep themselves from the artillery, going to cover with the canoes; and also when they traveled some shooting they let go, they stooped into the canoes.Bernardino de Sahagún General History of Things in New Spain Chapter XXX: Of how the bergantines made by the Spaniards in Tetzcuco came upon Mexico.
But the Mexicans when they saw, when they realized that the gunshots of cannon or arkbuz were right, they no longer walked in a straight line, but they went from one direction to the other making zigzag; they were on one side and on the other, they fled from the front. And when they saw that a cannon was going to be shot, they were thrown down, they tended, they squeezed to the earth.Miguel León Portilla, The Vision of the Lost Chapter 11: The Defensive Reaction of Mexicas
Gunpowder-based firearms began to be used either by locals or European expeditions, clashing since the 15th century until the early 20th century, as gunpowder and firearms were traded to Native Americans, mainly by the French and Portuguese, trying to weaken the influence of their European rivals (English and Spanish). At the end of the 19th century in confrontations between Native American forces against US forces, firearms did not bring a great strategic benefit, allowing the locals to win battles like Little Big Horn, where the Lakotas, Cheyennes, and Arapahoes defeated the 7th Cavalry Regiment. His defeat is partly attributed to his refusal to use Gatling guns.
Gunpowder-based firearms began to have considerable military advantage until the introduction of repeating firearms, developed at the turn of the century XIX, which were a determining factor in the culmination of the long wars against the Native Americans. This weapon was used against these populations mainly in the US, Mexico and Argentina, among others.
Each country developed its own gunpowder by varying the proportions of the mixture, the following table indicates some of the proportions adopted by different nations. Table 1
| Table of proportions of components of the powder of each nationality that produced it | |||
| United Nations | Salitre (KNO3) | Sulphur (S) | Coal (C) |
| Spain | 75% | 12.5% | 12.5% |
| France | 75% | 10% | 15% |
| Prussia | 71% | 11% | 16% |
| Saxony | 71% | 10% | 16% |
| England | 75% | 10% | 15% |
| Russia | 75% | 10% | 15% |
| Sweden | 75% | 10% | 15% |
| Austria | 75.5% | 10% | 11.5% |
| Belgium | 75% | 12.5% | 12.5% |
| Netherlands | 70% | 11% | 16% |
| Switzerland | 76% | 10% | 11% |
| Portugal | 75.7% | 10.7% | 13.6% |
| Italy | 75% | 10% | 15% |
| Turkey | 75% | 10% | 15% |
| United States | 76% | 10% | 14% |
| Persia | 75% | 12.5% | 12.5% |
| China | 61.5% | 15.5% | 23% |
Table 1 Table of proportions of gunpowder components by nationality that produced it.
Scientific facts
- Main pelvic ingredients
Chemically, coal and sulfur burn thanks to potassium nitrate, which is the oxidizer, since it supplies oxygen for combustion. Sodium nitrate can be used, but it is hygroscopic (it condenses on itself the humidity of the environment). There is also another gunpowder commonly used in the past, which instead of potassium nitrate, contains potassium chlorate (KClO3), whose use was common in pyrotechnics, but its use was gradually abandoned due to its high sensitivity. at temperature, friction and shock in favor of the more stable oxidant potassium perchlorate. Using sodium nitrate (Nitro de Chile), being more hygroscopic than potassium nitrate, it is necessary to decrease the quantity, while the sulfur dose must be increased.
A good experimental dose, in mass percent, is:
- 70% potassium nitrate;
- charcoal 17%;
- sulfur 13%.
You will then get the following reaction:
10 KNO3 + 5 S + 2 C7H3O => 5 Na2O + 3 H2O + 5 SO2 + 14 CO + 5 N2
For rapid combustion, charcoal produced by pyrolysis of wood at 500 °C is used. The best results are obtained with smut from Rhamnus Frangula, Solanum Mauritianum, house plum, Salix Caprea or Fraxinus americana. The sulfur and carbon must be reduced to a powder with particles smaller than 80 microns before mixing with a ball mill. Subsequently a mixture of sodium nitrate and alcohol is added and then everything is mixed in a blender, or in a mixer, to obtain a very homogeneous mixture. Finally, the mixture is dried at low temperature and gently reduced to a powder using a pestle. So you get a black powder that burns like a flash.
Potassium chlorate is not hygroscopic and works better than potassium nitrate, but combustion together with coal and sulfur is much faster, being almost explosive; for this reason it is used in pyrotechnics. The amounts of each component are: 50% KClO3, 35% carbon and 15% sulfur. Sulfur helps in combustion, because when it burns, it produces sulfur dioxide and trioxide, SO2 and SO3, and when it joins with the water molecules from it, it does not From combustion, but from humidity, sulfuric acid (H2SO4) and sulfurous (H2SO3) are produced ), which react violently with potassium chlorate, causing it to break down very quickly.
Although this type of gunpowder can still be found for the purposes described above, it was displaced by nitrocellulose or smokeless gunpowder in the last decade of the century XIX, totally replacing it for the notable advantages it had over the other.
Gunpowder deflagration
Even though this phenomenon seems to take place instantly, it is a proven fact that it occurs progressively and using a more or less long time, the time necessary, not only for the inflammation to spread to the entire mass of gunpowder that constitutes a load, but also for the total combustion of each grain. In order to calculate the amount of gunpowder burned in a given time, deducting from it that of the gases produced, it is necessary to take into account the greater or lesser speed, that is, the speed of each of the two phenomena that constitute the deflagration, which are: inflammation and combustion, understanding by inflammation the propagation of heat to the entire load, due to the expansive force of the gases at the high temperature with which they are produced from the first moment, and by combustion the combinations that have place between the elements of each grain or of all those that form the charge.
Inflammation can be produced by the shock of iron on iron, iron on brass and brass on brass, it is less easy hitting iron on copper and copper on copper; It is also produced by bronze on copper, iron on marble, quartz on quartz, lead on lead, lead on wood, very rarely by copper on wood and never by wood on wood, observing that the interposition of a sheet of paper between colliding bodies, favors inflammation. Gunpowder is also ignited by the rise in temperature. According to Piobert's experiences verified by Horsley, it is necessary for this that the temperature reaches 300° to 315° and according to Leygue and Champion it is enough to be 283° for hunting gunpowder and 293° for war powder. When the temperature rises gradually, the sulfur melts before reaching 300°, they unite the grains forming a paste: if the temperature continues to rise, the sulfur can vaporize and carry part of the coal with it, leading to the decomposition of the gunpowder without deflagrating, provided that the boiling temperature of the first is not reached. To determine the ignition temperature of gunpowder, Leygue and Champion used a copper bar that they heated at its extremities, isolating the source of heat by means of a screen to prevent radiation: they observed the temperature at different points on the bar. that were distant from each other a fixed magnitude, for which they used thermometers placed in cavities opened in it and filled with oil. When the temperature marked on the thermometers remained constant, they placed the gunpowder subjected to the experience at the coldest end of the rod, bringing it closer to the other end until inflammation or decomposition was verified.
The figures expressed should not be taken as precise, however if considered, as invariably indicating the ignition temperature of gunpowder; they serve only to mark a point around which the various kinds of gunpowder oscillate. The divergence that is noted in them must mainly be attributed to the state of grinding of the ingredients, the temperature necessary for inflammation being the higher the more perfect it is.
The contact of inflamed bodies is also the cause of the gunpowder igniting, in this case it is necessary that the temperature be very high, having observed that a flame can be touching the gunpowder for a few seconds without this phenomenon occurring.
The best and safest way to ignite gunpowder is by contact with igniting bodies.
Diverse are the causes that can favor or delay inflammation. When the gunpowder is wet, it slows down, which is due to the loss of heat to evaporate the water, and it may happen that if the humidity is high, it does not ignite and only slow combustion occurs: angular gunpowders are more easily flammable than round and also more non-blued than those that are.
A clearer example of the deflagration of gunpowder is in the firing of a bullet from a firearm.
Methods of identifying gunpowder in the hands of the shooter
There are several methods for the determination of gunpowder traces in the hands of the shooter, which what they do is use more effective reagents than diphenylamine, but they cannot solve the problems of false negatives, since they always depend on the shot whether or not it leaves traces of gunpowder in the hands of the shooter. What is done is to use other components of the gunpowder to obtain the positive, other than those elements, so generalized, in the environment, which can determine with absolute certainty that the shot has actually occurred, since this would constitute a false positive. The method that can determine the existence of gunpowder in general and not of some of its components, as is the case in any chemical or physical method, is not yet known.
The Maloney method is used to determine the components of gunpowder, brusine, that is, dimethoxystrychnine, in a sulfuric medium, which is more effective than diphenylamine.
Methods are also used that serve at the same time to determine the existence of gunpowder remains in other elements, such as clothing, skin in entrance holes (tattoos and/or smoking), or other elements where there is a projectile impact firearm at short distances.
The one carried out with a scanning electron microscope is a very modern method, which analyzes the chemical elements contained in the support to be analyzed, giving quantity and quality at the same time, that is, which chemical elements it contains and how many. It is an advisable method, with which you can analyze the residues extracted with the indicated elements, or the leather block in the area to be analyzed. We must clarify that although it is much more accurate than the previous methods, it also analyzes some components of the gunpowder, but not the gunpowder as a whole. The interesting thing about the subject is that if we know which are the components of the gunpowder that we want to analyze, we can know more exactly if it is gunpowder and what brand.
Mere contact with a firearm leaves residue on your hands, which can be detected by laboratory testing methods. Positive results do not unconditionally indicate that a person has discharged a firearm.
The metal residue contamination controls of the materials used in the laboratories were processed by several tests, and verifying that there is a possibility that in certain materials, such as water, nitric acid, plastic containers, glass, etc., can contribute to give a false positive, likewise a constant control of sources that promote contamination that is not from laboratories must be maintained).
The identification of traces of gunpowder can be carried out through three types of investigations:
Chemistry, Electronics and Microscopy
- Chemical determination. Brucin reaction: the solution is prepared at the time of use: 1 cm of pure sulfuric acid (HH2SO4) and a few drops of sorcery solution (C23H26N2O4) to 1 percent, in front of the suspected material, will give red or orange coloring in a positive case.
- Reaction of Wallenstein and Kober (1911) uses Guthman's reactive, which is a solution of diphelinmine sulfate (CC.12H11N) to one or more per cent, with concentrated sulfuric acid, which in positive case determines a dark blue coloration, which determines the presence of nitrites or nitrates.
- Corbozole or diphenyalmine reaction (C12H11N): in a jar they place 1 or 2 grams of defenilamine and 50cm of distilled water. It stirs well and then slowly add 50cm of concentrated sulfuric acid. If a test tube introduces some drops of this reactive and a drop of this reactive and a drop of the solution to examine, and then a cubic centimeter of concentrated sulfuric acid will get a green coloration, if there are nitrites and nitrates.
- Kunkel-Wetzel reaction. It is a method that can be applied when there is blood in place where and if there are traces of gunpowder deflagration, either in the hands or directly in the input hole of a firearm projectile, in the victim's body: a thong aqueous solution is used to three percent leaked. If the blood is not contaminated there is obscure brown coloration, instead, if there are traces of carboxyhemoglobin, product of powder deflagration, a living red color is noted.
- Friess's reaction. Destined to put a nitrite manifest: 0.50 centigrams of sulfanyl acid are dissolved (C6H7NO3S) in 150 cubic centimeters of acetic acid (CH)3COOH) to the tenth (solution a); they boil 10 centigrams of solid anaftilamine of 20 cubic centimeters of water; and separates the colorless solution of residue, which presents a violet blue coloration and adds 150 cubic centimeters of acetic acid to the tenth (solution b). At the time of use, the liquid is added to investigate a small amount of the solution to, it is heated to 70-80°. And then add a number of solution b. It is enough that the solution contains the thousandth of nitrates so that a reddish colour appears at the minute.
- Peccini and Debassys reaction: Use ferrous sulfate (FeSO(4) in a sulfur. When there are products nitreated in the solution to be examined, a pink coloration appears.
- Sulfanyl acid reaction (C6H7NO3S) and alpha-naftol-amico-athetic; or reaction of the F.B.I.: Photo paper desensitized by removal of the sensitive film, remaining only the layer of the jelly. Paper is treated with a solution to half percent of distilled water sulfanyl acid and is then dried. After acting on the same paper a solution to the half percent of alfanaftolamine of methyl alcohol and drying again. Suspect tissue is then applied against the treated paper. Nitrites from the gunpowder are transferred to the paper by pressure on the tissue with a hot iron plate moistened in a solution to 25% acetic acid in distilled water
- The spectrophotometry of atomic absorption, which makes it possible to identify and quantify the amounts of barium and antimony, is described as a high probability. With Richard Saferstein’s words, “The demonstration of high levels of barium and antimony in the hands of the suspect is a strong presumption that the person shot or had the weapon used in his hands.
- The electronic sweeping microscopy coupled with an X-ray analyzer is appreciated as of certainty, as it identifies the residues of the shot by its morphology and composition.
Contenido relacionado
Alfonso XII of Spain
Sasanian Empire
I fight




![Cañón de mano de bronce de la Dinastía Yuan 1332; describe una mezcla de seis partes de azufre y seis partes de salitre y una parte de Aristolochia (de donde se obtenía el carbón).[4]](https://upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Bronze_cannon_of_1332.jpg/357px-Bronze_cannon_of_1332.jpg)