Graphite
Graphite is one of the allotropic forms in which carbon can occur in nature. Other forms are diamond, chaoite and lonsdaleite. At atmospheric pressure and room temperature the most stable polymorph is graphite. However, the transformation of diamond into graphite is so extremely slow that it is only appreciable on a geological scale.
It was named by Abraham Gottlob Werner in 1789. The term graphite derives from the Greek γραφειν (graphein) meaning "to write," as it is mainly used to create the tips of pencils. It is also known by the common name of plombagina. It was formerly called "molibundasna" (not to be confused with the mineral molybdenum).
Abundance
Graphite appears in metamorphic rocks as a result of the reduction of carbon compounds from sedimentary rocks during metamorphism. It is also found in igneous rocks and meteorites. The minerals associated with graphite are quartz, calcite, mica, and tourmaline.
In meteorites, graphite occurs with troillite and silicate minerals. Small crystals of graphite in meteoric iron are called cliftonite. Some microscopic grains have distinctive isotope compositions, indicating that they formed before the Solar System. They are one of about 12 types of minerals known to predate the Solar System and have also been detected in molecular clouds. These minerals were formed in ejecta when supernovae exploded or low- to intermediate-sized stars shed their outer envelopes at the end of their lives. Graphite may be the second or third oldest mineral in the Universe.
Although it can be produced artificially, from amorphous carbon, there are many natural deposits of this mineral. It is found in metamorphic rocks, in which the processes associated with its formation have transformed the carbon present in the organic matter contained in the original rock. The main exporting countries of extracted graphite are, in order of tonnage: China, Mexico, Canada, Brazil and Madagascar.
Structure
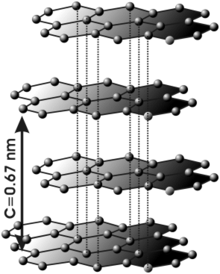
Graphite crystallizes in the hexagonal system. In graphite, the carbon atoms present sp2 hybridization, this means that it forms three covalent bonds in the same plane at an angle of 120° (hexagonal structure) and that an Π orbital i> perpendicular to that plane is free (these delocalized orbitals are essential to define the electrical behavior of graphite). The covalent bond between the atoms in a shell is extremely strong. However, the bonds between the different layers are made by Van der Waals forces and interactions between the Π orbitals, and are much weaker. It could be said that graphite is made up of superimposed sheets of graphene. This lamellar structure makes graphite a markedly anisotropic material.
Properties
Graphite is black and gray in color with a metallic luster, refractory, and exfoliates very easily. In the direction perpendicular to the layers, it has a low electrical conductivity, which increases with temperature, thus behaving like a semiconductor. Throughout the layers, the conductivity is greater and increases proportionally to the temperature, behaving like a semi-metallic conductor. Although both graphite and diamond are made up exclusively of carbon atoms, graphite is very soft and opaque, while diamond is the hardest mineral on the Mohs scale and also allows light to pass through it. These marked physical differences are due exclusively to the different crystal lattices or lattice on which the carbon atoms are arranged in graphite (carbon atoms at the vertices of hexagonal prisms) and in diamond (the crystal lattice is made of regular tetrahedrons). whose vertices are carbon atoms).
Applications
- The graphite mixed with a pasta is used to manufacture the pencil mine.
- It is used as a component of refractory bricks, crucibles, etc.
- By sliding the layers easily into the graphite, it turns out to be a good solid lubricant.
- It is used in the manufacture of various parts in engineering, such as pistons, joints, washers, bearings, etc.
- Due to its electrical conductivity and chemical resistance it is used to manufacture electrodes. It also has other electrical applications, such as the coals of an engine (scobillas), which come into contact with the collector.
- Used in nuclear reactors, such as moderator.
Artificial synthesis of graphite
In 1893, Charles Street of Le Carbone discovered a process for making artificial graphite. In the mid-1890s, Edward Goodrich Acheson (1856-1931) accidentally invented another way to produce synthetic graphite after synthesizing carborundum (silicon carbide, or SiC). He discovered that superheating carborundum, unlike pure carbon, produced almost pure graphite. By studying the effects of high temperatures on carborundum, he discovered that silicon vaporizes at about 4,150 °C (7,500 °F), leaving carbon in graphite. This graphite became valuable as a lubricant.
Acheson's technique for producing silicon carbide and graphite is called the Acheson process. In 1896, Acheson received a patent for his method of synthesizing graphite, and commercial production began in 1897. In 1899, the Acheson Graphite Co. was formed.
Synthetic graphite can also be made from polyimide and is marketed as such.
Graphite Intercalation Compounds
Different molecules or ions can penetrate the graphite layers. For example, potassium can donate an electron to graphite, leaving the potassium ion, K, between the layers. This electron contributes to increase the conductivity that carbon had.
Different intercalation compounds with different stoichiometries and different species can be prepared. In some cases the resulting conductivity is higher, as in the case of potassium, and this is what generally occurs, but in others, such as fluorine, it is to a lesser extent.
Other related forms
There are other forms called amorphous carbon that have a structure related to that of graphite:
- Charcoal and activated carbon
- Black smoke
- Carbon fibre
- Pyrolytic carb
- Passive fire protection
- Grapheno
- Fuller.
- Mineral elements
Contenido relacionado
Alpha particle
Lanthanides
Bronze