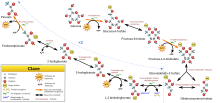
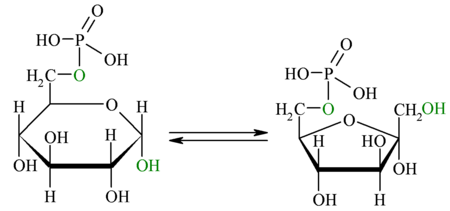
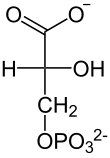
Glycolysis
| Global glycolysis reaction |
|---|
 ⇒ ⇒ {displaystyle Rightarrow } ⇒ ⇒ {displaystyle Rightarrow } 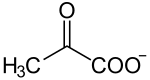 + +  |
| α-D-glucosa + 2NAD+ + 2ADP + 2Pi == 2(piruvate) + 2NADH + 2ATP + 2H+ + 2H2O |
Glycolysis (from the Greek glycos, sugar and lysis, breakdown, destruction, transformation) is the metabolic pathway responsible for oxidizing glucose in order to obtain energy for the cell. It consists of 10 consecutive enzymatic reactions that convert glucose into two molecules of pyruvate, which is capable of following other metabolic pathways and thus continuing to deliver energy to the body. This route is carried out both in the absence and in the presence of oxygen, defined as an anaerobic process in this case.
The most common and well-known type of glycolysis is the Embden-Meyerhof pathway, first explained by Gustav Embden and Otto Fritz Meyerhof. The term may include alternative pathways, such as the Entner-Doudoroff pathway. However, the term "glycolysis" is often used synonymously with the Embden-Meyerhof pathway. The Krebs cycle continues. It is the initial pathway of carbohydrate catabolism.
Discovery
The first informal studies of glycolytic processes began in 1860, when Louis Pasteur discovered that microorganisms are responsible for fermentation, and in 1897 when Eduard Buchner found that a certain cell extract could cause fermentation. The next great contribution was made by Arthur Harden and William Young in 1905, who determined that for fermentation to take place, a cellular fraction of high molecular mass and heat-sensitive (enzymes) and a cytoplasmic fraction of low molecular mass and heat-resistant (ATP, ADP, NAD+ and other coenzymes). The details of the track itself were determined in 1940, with a breakthrough by Otto Meyerhoff and a few years later by Luis Leloir. The major difficulties in determining the intricacies of the pathway were the short life and low concentrations of the intermediates in the rapid glycolytic reactions.
In eukaryotes and prokaryotes, glycolysis occurs in the cytosol of the cell. In plant cells, some of the glycolytic reactions are also found in the Calvin cycle, which occurs within chloroplasts. The broad conservation of this pathway includes the phylogenetically oldest organisms, and for this reason it is considered one of the oldest metabolic pathways.
Overview
During glycolysis, a net yield of two ATP molecules is obtained; ATP can be used as a source of energy to perform metabolic work, while NADH can have different destinations. It can be used as a source of reducing power in anabolic reactions; if there is oxygen, it can be oxidized in the respiratory chain, obtaining 5 ATP (2.5 for each NADH); if there is no dioxygen, it is used to reduce pyruvate to lactate (lactic fermentation), or to CO2 and ethanol (alcoholic fermentation), without obtaining additional energy.
Glycolysis is the fastest way to get energy for a cell and, in carbohydrate metabolism, it is usually the first route that is used. It is structured in 10 enzymatic reactions that allow the transformation of a glucose molecule to two pyruvate molecules through a catabolic process.
Glycolysis is one of the most studied pathways, and is generally divided into two phases: the first, for energy expenditure, and the second, for obtaining energy.
The first phase consists of transforming a glucose molecule into two glyceraldehyde molecules (a low energy molecule) by using 2 ATP. This makes it possible to duplicate the results of the second phase of obtaining energy.
In the second phase, glyceraldehyde is transformed into a high-energy compound, whose hydrolysis generates a molecule of ATP, and since 2 molecules of glyceraldehyde were generated, two molecules of glyceraldehyde are actually obtained. ATP. This energy gain is achieved by coupling a strongly exergonic reaction after a slightly endergonic one. This coupling occurs once more in this phase, generating two pyruvate molecules. In this way, in the second phase, 4 ATP molecules are obtained.
After Reactions
After a glucose molecule is transformed into 2 pyruvate molecules, the conditions of the environment in which it is found will determine the metabolic pathway to follow.
In aerobic organisms, pyruvate will continue to be oxidized by the enzyme pyruvate dehydrogenase and the Krebs cycle, creating intermediates such as NADH and FADH2. These intermediates cannot cross the mitochondrial membrane, and therefore use exchange systems with other compounds called shuttles. The best known are the malate-aspartate shuttle and the glycerol-3-phosphate shuttle. The intermediaries manage to deliver their equivalents to the interior of the mitochondrial membrane, and which will then pass through the electron transport chain, which will use them to synthesize ATP.
In this way, up to 30 moles of ATP can be obtained from 1 mole of glucose as a net gain.
However, when the cells do not have mitochondria (eg, erythrocytes) or when they require large amounts of ATP (eg, muscles when exercising), pyruvate undergoes fermentation that allows obtaining 2 moles of ATP for each mole glucose, so this pathway is inefficient compared to the aerobic phase of glycolysis.
The type of fermentation varies with respect to the type of organisms: in yeasts, alcoholic fermentation occurs, producing ethanol and CO2 as final products, while in muscle, erythrocytes, and some microorganisms, lactic fermentation occurs, resulting in lactic acid or lactate.
Functions
The functions of glycolysis are:
- The generation of high-energy molecules (ATP and NADH) as a source of cell energy in aerobic breathing processes (oxygen pressure) and fermentation (oxygen failure).
- The generation of rookie that will pass to the cycle of Krebs, as part of aerobic breathing.
- The production of 6- and 3-carbon intermediaries that can be used in other cell processes.
Stages of glycolysis
Glycolysis is divided into two main parts and ten enzymatic reactions, which are described below.
Energy Expenditure Phase (ATP)
This first phase of glycolysis consists of transforming a glucose molecule into two glyceraldehyde molecules.
Energy benefit phase (ATP, NADH)
So far only energy (ATP) has been consumed, however, in the second stage, glyceraldehyde is converted to a high-energy molecule, where finally the final benefit of 4 ATP molecules will be obtained.
| 6.o step: glyceraldehyde-3-phosphate dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| This reaction consists of oxidizing the glyceraldehyde-3-phosphate using NAD+ to add a phosphate ion to the molecule, which is made by the glyceraldehyde-3-phosphate dehydrogenase enzyme in 5 steps, and in this way increase energy of the compound.
Technically, the aldehyde group is oxidized to an acil-phosphate group, which is a derivative of a phosphate carboxyl. This compound has a very high hydrolysis energy (near to 50 kJ/mol) so the reactions process is started to recover the ATP later. While the aldehyde group is oxidized, the NAD+ is reduced, which makes this reaction a redox reaction. The NAD+ is reduced by the incorporation of some [H+] resulting in a neutral charge NADH molecule. |
| ||||||||
| 7.o step: fosfoglicerato quinasa
See also: Fosfoglicerato quinasa | |||||||||
| In this step, the quinasa phosphoglycerate enzyme transfers the phosphate group of 1.3-bisphoglycerate to an ADP molecule, thus generating the first ATP molecule of the pathway. Since glucose was transformed into 2 glyceraldehyde molecules, a total of 2 ATPs are recovered at this stage. Note that the enzyme was named by the reverse reaction to the shown, and that it operates in both directions.
Steps 6 and 7 of glucolysis show us a case of coupling reactions, where an energetically unfavourable reaction (step 6) is followed by a very favorable energetic reaction (step 7) that induces the first reaction. In other words, as the cell remains in balance, the decline in reserves of 1.3-bisphoglycerate pushes the GAP dehydrogenase enzyme to increase its reserves. The quantification of free energy for the coupling of both reactions is about -12 kJ/mol. This way to get ATP without the need for O2 is called phosphorylation at substrate level. |
| ||||||||
| 8th step: mutated phosphoglycerate
See also: Mutual fosfoglicerate | |||||||||
| Separate isomeriza the 3-phosphoglycerate from the previous reaction giving 2-phosphoglycerate, the enzyme that catalyzes this reaction is the mutase phosphoglycerate. The only thing that happens here is the shift from the C3 to C2 phosphate position. They are similar and therefore reversible energies, with a variation of free energy close to zero. |
| ||||||||
| 9th Step: Enolasa
See also: Enolasa | |||||||||
| Enolase enzyme promotes the formation of a double link in the 2-phosphoglycerate, eliminating a water molecule formed by the hydrogen of C2 and the OH of C3. The result is phosphoenolpiruvate. |
| ||||||||
| 10.o step: chinese rookie
See also: Piruvato quinasa | |||||||||
| Fosfoenolpiruvato dephosphorylation, obtaining pyruvate and ATP. Irreversible reaction mediated by the chinese pyruvate. The enzyme piruvato quinasa is dependent on magnesium and potassium. Free energy is -31.4 kJ/mol, therefore the reaction is favorable and irreversible. |
| ||||||||
The total yield of the glycolysis of a single glucose (6C) is 2 ATP and not 4 (two for each glyceraldehyde-3-phosphate (3C)), since 2 ATP are consumed in the first phase, and 2 NADH (which will leave the Nc electrons in the electron transport chain to form 3 ATP for each electron). With the pyruvate molecule, through an intermediate oxidation step called oxidative decarboxylation, through which pyruvate passes into the mitochondria, losing CO2 and an electron that oxidizes NAD+, which becomes NADH plus H+ and gaining a CoA-SH (coenzyme A), forming acetyl-CoA thanks to the enzyme pyruvate dehydrogenase, one can enter the Krebs cycle (which, together with the electron transport chain, is called respiration).
Regulation
The Pasteur effect
The Pasteur effect is the visualization of the power that O2 possesses in fermentation mediated by yeast, which was discovered by Luis Pasteur when observing the relationship between the fermentation rate and the existence of air. He determined that these were inversely related, and further noted that under aerobic conditions, yeast cells increased and fermentation decreased.
In this way, the Pasteur effect was one of the first observations that someone made to the process of glycolysis indirectly, but observing that the primary metabolism of glucose could be carried out with the presence or absence of oxygen, and that in this last occurs alcoholic fermentation.
Substrate regulation
The plasma membrane of cells is permeable to glucose. To carry it inside it, it uses special transporters called GLUT, of which there are no different types and some are specialized for each cell.
Regulation of enzyme activity
Glycolysis is regulated enzymatically at the three irreversible points of this pathway, that is, in the first reaction (G → G-6P), by means of hexokinase; in the third reaction (F-6P → F-1,6-BP) by means of PFK1 and in the last step (PEP → Pyruvate) by pyruvate kinase.
- La hexoquinas is an unimportant regulatory point, as it is inhibited when there is a lot of G-6P in muscle. It is a little important point as the G-6P is used for other tracks.
- La fosfofructoquinasa-1 is the main enzyme of glucolysis regulation, it acts as a water key, if it is active catalyses many reactions and gets more fructosa-1,6-bisphosphate, which will allow the following enzymes to transform a lot of pyruvate. If inhibited, low concentrations of product are obtained and therefore little pyruvate is obtained. This enzyme is controlled by alostheric regulation: on the one hand it is activated by high concentrations of ADP and AMP, inhibiting in abundance of ATP and citrate, and on the other it is activated in the presence of a PFK2-generated regulator that is fructose-2.6-bisphosphate (F-2.6-BP), which is not a metabolite or glucolysis or gluconeogenesis, but a regulator of both pathways that reflects the level of glucagon in blood.
- The logic of inhibition and activation are the following:
- ATP: inhibits this enzyme because if there is a high concentration of ATP then the cell does not need to generate more.
- Citrate: If the citrate concentration is high the Krebs Cycle goes slower than the substrate (acetyl-CoA) comes to degrad, and the glucose concentration will be higher. In the Krebs Cycle there is a lot of NADH and FADH2, for them to work they have to be reoxidated in the electronic transport chain creating proton gradient, if the gradient is not spent the coenzymes are not reoxidated and the Krebs Cycle stops.
- AMP, ADP: the high concentration of these molecules implies that there is a lack of ATP, so it is necessary to perform glucolysis, to generate pyruvate and energy.
- La piruvatoquinas is regulated differently according to the tissue in which you work, but in the liver it is inhibited in the presence of ATP and Acetil Coenzima-A (Acetyl-CoA), and it is activated again before the F-1,6-BP and the concentration of phosphoenolpiruvate.
Hormonal regulation
When blood glucose rises after a meal, beta cells in the pancreas stimulate insulin production, which in turn increases glucokinase activity in hepatocytes.
High glucagon concentrations and low insulin levels decrease the intracellular concentration of fructose-1,6-bisphosphate. This results in decreased glycolysis and increased gluconeogenesis.
Glucose production
Gluconeogenesis is the anabolic pathway by which the synthesis of new glucose takes place from non-glycosidic precursors (lactate, pyruvate, glycerol and some amino acids). It takes place mainly in the liver, and to a lesser extent in the renal cortex. It is stimulated by the hormone glucagon, secreted by the α (alpha) cells of the islets of Langerhans of the pancreas and inhibited by its counterregulator, the hormone insulin, secreted by the β (beta) cells of the islets of Langerhans of the pancreas. stimulates the catabolic pathway called glycogenolysis to break down stored glycogen into glucose and thus increase blood glucose (blood sugar).
From an enzymatic point of view, producing glucose from lactic acid or pyruvate costs more than its phosphoric degradation produced.
The overall equation is:
| 2 pyruvato + 4 ATP + 2 GTP + 2 NADH + 2 H+ + 4 H 2O → glucose + 4 ADP + 2 GDP + 6 Pi + 2 NAD+ |
Glycolysis in plants
Plants have the ability to carry out photosynthesis, and among the by-products of this process is glucose. This is used by plants, among many things, as a source of energy in the respiration process, which, unlike photosynthesis, is carried out independently of light. As plants breathe, they absorb oxygen from the air and expel carbon dioxide and water vapor. The exchange of substances is carried out by the stomata; openings that act as gates in the plants that also have the characteristic of closing in the event of an excessive decrease in atmospheric vapor.
Contenido relacionado
Archaea
Aloe vera
Huntington's disease