Glass
Glass is a hard, brittle, transparent and amorphous inorganic material found in nature, although it can also be produced by humans. Artificial glass is used to make windows, glasses, bottles and a wide variety of products. Glass is an amorphous type ceramic material.
Glass is obtained at about 1500 °C from silica sand (SiO2), sodium carbonate (Na2CO3) and limestone (CaCO3).
In some Spanish-speaking countries, the term «cristal» is frequently used as a synonym for glass, although it is strictly incorrect because glass is an amorphous solid (its molecules are arranged irregularly) and not a solid crystalline.
History
Glass in ancient times
Glass can be found naturally in the form of obsidian, which is produced when high-silica volcanic lava is cooled rapidly so that crystals do not form. Obsidian has been used since prehistoric times mainly to make sharp objects because when it fractures it forms sharp edges, with the first evidence in present-day Kurdistan around 12,500 BC. c.
Pliny the Elder (I century), in his Natural History, recounts that the glass was discovered on the banks of the river Belus, in Phoenicia, whose sand, due to its great purity and high silica content, was used throughout antiquity for the production of glass. The legend was later recounted by the alchemist Georg Bauer in his treatise De re metallica:
Some merchants heading for Egypt to sell nitro (potassium nitrate, KNO3) stopped for dinner. Since there were no stones to place their pots, they decided to use some pieces of the mineral. They warmed their food, ate and disposed to sleep. The next morning they saw astonished that the stones had melted and reacted with the sand to produce a hard and brilliant material, the glass.
Actually, man learned how to make glass a long time before, probably from experimenting with ceramic glazes or from residual slag from metallurgical smelting. The first examples have been found in Egypt and the coast of present-day Lebanon around 2500 BC. C. in the form of beads or beads and occasionally containers and ingots. It is believed that the production of glass was kept as a secret reserved for the production areas, artisans from other areas had to limit themselves to buying ingots to make their pieces. Glass made during this period was not transparent and tried to imitate the appearance of semi-precious stones. In the tombs of the pre-dynastic period of Egypt, in the Naqada cultures (3500-3200 BC). Beads from necklaces and remains of ceramics made with faience are frequently found. Although not actual glass, faience is a type of quartz-rich ceramic that exhibits a glossy finish due to vitrification of its surface.
It is likely that Asian artisans established glass manufacturing in Egypt, where the first vessels produced during the reign of Tuthmosis III (1504-1450 BC) came from. Glass making flourished in Egypt and Mesopotamia until 1200 B.C. C. and subsequently ceased almost completely until the IX century B.C. C. when production resumed after developing techniques to create transparent glass. Egypt produced a clear glass, which contained pure silica and as an alkaline element they used natron (hydrated sodium carbonate: Na2CO3 10H2O) extracted from mineral deposits or sodium carbonate obtained by calcining various plants, especially those that grow in brackish water. They colored it blue and green. During the Hellenistic period, Egypt became the main supplier of glassware to the royal courts. New manufacturing techniques such as the use of molds to create reliefs and millefiori, in which fragments of colored glass bars are fused together to form mosaic-like patterns, were developed in this period.
However, it was on the Phoenician shores that the important discovery of blown glass took place in the I century B.C. C. Previously the containers were created in a very laborious way, the molten glass was extended in cords on a central core of clay and sand that served as a mold. Once cooled, the mold was removed and the piece was finished with polishing techniques similar to those used for stone.
During Roman times, glass manufacturing spread throughout the Empire, from Rome to Germany. The production of raw glass was often carried out in different places from where it was worked. At this time it was discovered that adding manganese oxide could clarify the glass and they also developed the recycling of Roman glassware. The name in Spanish also comes from the Romans, since the natural coloration of glass was green, a name that it was pronounced as viride, or viridus; hence the name viridium or glass. These advances led to the development of large-scale industry from the I century, especially in Alexandria, glass becoming a much more affordable and widespread product than in earlier times.
In India, the oldest glass ever found is a red bead dating back to the 18th century a. C., during the Indus Valley civilization. Glass from later periods has been found but it is not until the III century BCE. C. which is found in appreciable quantities, and its use became widespread from the I century d. c.
In ancient China, glass arrived around the Spring and Autumn period, in the form of pieces imported from Central Asia. Later there is archaeological evidence of glass bead production in the Warring States period, around the early V century BCE. C. Subsequently, in the Han period (206 BC-220 AD) the production became more diverse to decline later and reappear around the V. The composition of Chinese glass from this period is very different from that of the rest of the world, with a formulation based on barium oxide and lead. The proportion of BaO is 5-15%, it has been speculated that this material provided a turbidity to the glass that made it resemble jade. Glass remained a by-product throughout Chinese history, far behind porcelain and other more prized materials.
Glass in the Middle Ages
Glass in Islamic countries, between the 8th and 14th centuries, had its peak in the Near East. The ancient Sasanian tradition of glass carving was continued by Muslim artisans who made high-relief decorated vessels, many with animal motifs, and high-quality colorless glass with wheel-carved designs. The fire enamel technique and the gilding technique increased the decorative possibilities, highlighting the glass craftsmen of Aleppo and Damascus. From Egypt comes the discovery of glazed colors with brilliant metallic effects, both in ceramics and glass. The lamps of the mosques and other vessels of daily use were painted with geometric motifs typical of Islam. Its shapes and decorations influenced subsequent western production, highlighting those of Venice and Spain.
In Northern Europe and Great Britain they continued to produce glass utilitarian objects. Common glass type Waldglas (from German, 'forest glass') continued to be made in Europe until the modern era. However, the most important production in this material during the Middle Ages were glass mosaics in Mediterranean Europe and stained glass in the north. The mosaics were made with glass tiles, which were cut from glass blocks. Documents from the VI century refer to stained glass windows in churches, although the earliest surviving examples date from the XI. The most appreciated were made during the 13th and 14th centuries, mainly in France and England. The glass was colored or laminated already colored by adding metal oxides to the mixture, and then cut. The details were painted on the glass with an enamel. The pieces were held together with a lead net known as leaded. lowercase">XIX.
From the Renaissance to the 18th century
- The Venetian glass
The oldest known "Venetian glass" dates from the 15th century, although glass had been made in Venice since the century X. Centered on the island of Murano, the Venetians dominated the European market until the year 1700. The most important contribution was the production of a highly ductile hard and refined soda glass. Known as "cristallo", it was colorless, highly transparent, very similar to rock crystal. They were also made of colored and opaque glass. Towards the end of the 16th century the vessels became lighter and more delicate. They developed a type of glass filigree that would be widely imitated. It consisted of incorporating strands of opaque white glass inside a transparent glass, which produced the effect of lace.
Many different styles of glass chandeliers arose in Murano as well, although it was the factory in Nevers, France, which became most famous during the century XVII. The practice of diamond engraving, a technique of Dutch artisans of the 17th century, achieved elaborate designs.
Glassmakers in Europe tried to copy the techniques and decorations of the Venetians. The information spread with the book The Art of Glass (1612) by Antonio Neri, and also by the Venetian glass blowers, because although a law prohibited glass craftsmen from leaving Venice and divulging the secrets of his art, many settled in other European countries. Each country developed its imitations. Italian influence waned in the 17th century century, as new methods of glass-making emerged in Germany and England.
Vitreous state
Traditionally it has been considered that matter could appear in three forms: solid, liquid and gaseous. New means of investigating its intimate structure —particularly during the XX century— have revealed other forms or states in which matter can be presented. For example, the mesomorphic state (a liquid form with its smectic, nematic and cholesteric phases), the plasma state (or plasmatic state, typical of ionized gases at very high temperatures) or the vitreous, among others.
Bodies in the vitreous state are characterized by having a solid appearance with a certain hardness and rigidity and that, when faced with moderate external stresses, deform in a generally elastic manner. However, like liquids, these bodies are optically isotropic, transparent to most of the electromagnetic spectrum of visible radiation. When its internal structure is studied through means such as X-ray diffraction, it gives rise to diffuse diffraction bands similar to those of liquids. If they are heated, their viscosity gradually decreases —like most liquids— until they reach values that allow them to deform under the action of gravity, and for example, take the shape of the container that contains them as true liquids. However, they do not present a clearly marked point of transition between the solid and liquid state or "melting point".
All these properties have led some researchers to define the glassy state not as a different state of matter, but simply as that of a subcooled liquid or a liquid with such a high viscosity that it confers appearance of a solid without being one. This hypothesis implies the consideration of the glassy state as a metastable state to which a sufficient activation energy of its particles should lead to its equilibrium state, that is, that of a crystalline solid.
In support of this hypothesis, the experimental fact is adduced that, when a body is heated in the vitreous state until obtaining a clearly liquid behavior (at a sufficiently high temperature so that its viscosity is less than 500 poises, for example), if it cools slowly and carefully, providing it with the necessary activation energy for the formation of the first solid corpuscles (seeding of microcrystals, presence of activating surfaces, nucleation catalysts, etc.) it usually solidifies, giving rise to the formation of sets of true solid crystals.
Everything seems to indicate that bodies in the vitreous state do not present a determined internal arrangement, as occurs with crystalline solids. However, in many cases an ordered disorder is observed, that is, the presence of ordered groups that are distributed in space in a totally or partially random manner.
This has led different researchers to propose various theories about the internal structure of the glassy state, both geometric, based on both atomic and energy theories.
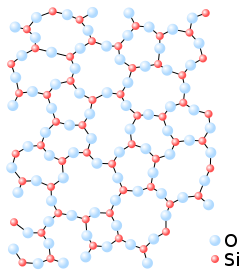
According to the geometric atomic theory, in crystallized solid silica, the silicon atom is surrounded by four oxygen atoms located at the vertices of a tetrahedron, each of which binds the atoms neighboring silicon. A plan view of this arrangement is schematized in figure 1, in which the fourth oxygen would be above the plane of the page. When this silica passes into the glassy state, the tetrahedral arrangement continues to be maintained at the individual level of each silicon atom, although the bonds between oxygen and silicon atoms are made in an apparent disorder, which nevertheless maintains an initial unitary organization (see figure 2).
However, none of these theories is sufficient to explain the complete behavior of vitreous bodies, although they can serve to answer, in specific and well-determined cases, some of the questions that arise.
Substances capable of presenting a glassy state can be both inorganic and organic in nature, among others:
- Chemical elements: Yes, Se, Au-Si, Pt-Pd, Cu-Au.
- Oxides: SiO2B2O3, P2O5and some of their combinations.
- Compounds: As2S3GeSe2, P2S3, BeF2, PbCl2, AgI, Ca(NO3)2.
- Silicones (substances considered as semi-organic)
- Organic polymers: such as glycols, sugars, polyamides, polystyrene or polyethylene, etc.
Common glass
Vitreous silica
Silica is an oxide of silicon with the chemical formula SiO2. It occurs in a crystalline solid state under different enantiotropic forms. The best known are quartz (the most frequent and stable at room temperature), cristobalite and tridymites. In addition to these forms, up to twenty-two different phases have been identified, each of them stable from a perfectly determined temperature.
When quartz is slowly heated, it goes through different enantiotropic forms until it reaches its melting point at 1723 °C. At this temperature a colorless and highly viscous liquid is obtained which, if cooled relatively quickly, becomes a vitreous substance that is usually called quartz glass.
This quartz glass presents a set of highly useful properties that can be applied in multiple disciplines: in scientific and technological research, in domestic life and in general in all types of industry. The following stand out as most relevant:
- Great resistance to attack by chemical agents, so it is widely used as laboratory material. It is only attacked, in an important way at room temperature, by fluoric acid in its different forms (gaseous or dissolution). At temperatures above 800 °C it reacts to appreciable speeds with alkaline or alkaline-therral salts, in particular with sodium salts, such as carbonate or sodium sulfate.
- While its ambient temperature density is relatively high (2.2 g/cm3) its medium linear dilation coefficient at temperatures below 1000 °C is extremely small: it is located at 5.1•10-7K-1, which allows, for example, to warm it to the red and submercate it abruptly in water, without fracture. The number of applications this property raises is high.
- Its refraction index to visible electromagnetic radiation is 1,4589, which makes it suitable for optical instruments in general.
- Its electric resistivity is the order of the 1020ohm·cm in normal conditions which makes it one of the best known electric insulators, with all the applications that are derived from it in the modern industry.
- The absorption of the electromagnetic radiation from quartz glass shows great transparency in visible light as well as in the bands corresponding to the ultraviolet spectrum, which makes it especially suitable for the manufacture of lamps and other generating instruments of this type of radiation.
Other properties, however, make it difficult to make and use. In particular, the following:
| Temperature | Viscosity |
| °C | μ (poises) |
| 1800 | 107.21 |
| 2000 | 106.10 |
| 2200 | 105,21 |
| 2400 | 104.50 |
| 2600 | 103,90 |
| 2800 | 103,40 |
- The melting point of the crystallized silica depends on the enanciotropic variety in question. For stable variety from 1470 °C (alpha-cristobalite) this is 1723 °C. These are temperatures that cannot be easily reached, except in highly specialized facilities. For this reason, the manufacture of quartz glass It's always been weird and expensive. Industrially, its production is quite limited if compared to other types of glass.
- Its viscosity in vitreous state presents a great variation with temperature, passing from values above 107 poises (total solid aspect) below 1800 °C, at 103.5 poises at 2758 °C (grass and mouldable look).
- Viscosities take such extremely high values that must be expressed as powers of ten. In general, the viscosities of the glass are usually given in the form of its decimal logarithm. To obtain the quartz glass it is necessary to start from a crystallized quartz of great purity, finely ground, which is subjected to high temperatures. The liquid that is obtained presents a lot of tiny bubbles of ocluid air between the grains of quartz, which give it a milky, translucent look, which is usually called gres de quartz and whose applications as a chemical attack resistant container or sudden temperature changes are frequent. However, it is totally useless for applications where great transparency is needed (UVA lamps, quartz lamps and optics in general). For the latter it is necessary that during the fusion process these occlusive gaseous bubbles can be detached. In order for this detachment to be effective under atmospheric pressure and at an industrially applicable speed, it would be necessary for the liquid to present a viscosity below 200 poises, which in the case of liquid silica would involve temperatures of the order of 3600 °C. In practice to degasify silica glass the quartz is melted at temperatures close to 2000 °C in containers where the vacuum is made, complicating the technology of its production and therefore increasing the product.
- The resistance to traction in pure state, in normal conditions and with a surface perfectly free of all fissure, is about 60 kbar. This great resistance (better than that of steel) is strongly diminished by imperfections on the surface of the object, however small they are.
- Its Young to 25 °C module is 720 kbar and 290 kbar torsion. When subjected to a mechanical traction effort at temperatures close to the environment, it behaves like a perfectly elastic body with a function enlargement/forcement linear, but with virtually no plastic area close to its breaking limit. This property, coupled with the mechanical resistance to the above-mentioned traction, makes it a fragile product. By hitting it, or deforming it elasticly and its shape is not altered or, if it exceeds its elasticity limit, it is fractured.
Sodium silicate
The most common sodium salts have melting points below 900 °C. When an intimate mixture of finely divided quartz is heated with a salt of these alkali metals, for example Na2CO3, at a temperature above 800 °C, it is obtained initially a fusion of the alkaline salt, whose liquid surrounds the quartz grains, producing a series of reactions that can be included in the following result:
Yes2 (s) + Na2CO3 (s) → → {displaystyle rightarrow }Na2Yes3 (s) + CO2 (g) Δ Δ {displaystyle Delta }H = -5,12 kcal/mol
This slightly exothermic reaction gives off gaseous carbon dioxide -which bubbles through the molten mass- and leads to a first sodium silicate, with a melting point of 1087 °C.
According to thermodynamics, the mixture of two substances with different melting points presents a "liquidus point" that lies between those of the two substances in contact. In this way, the mixture of silica and sodium silicate formed gives rise to a product of SiO2 and silicates, already in a liquid state at temperatures that do not exceed 1200 °C, far from more than 2000 °C required to prepare the quartz glass.
The product thus obtained is commonly given the generic name sodium silicate, although this name identifies a group of products derived from the fusion of quartz with sodium salts (generally carbonates) in different proportions of one component and another. Industrially, sodium silicates are prepared with molar proportions of each component located between:
- 3,90 mills of SiO2 / 1 mol of Na2O and 1,69 mills of SiO2 / 1 mol of Na2O
- Note
- The schiometric proportion of a pure sodium metalicate would be 1 mol of SiO2 / 1 mol of Na2O
These sodium silicates have a glassy, transparent and very brittle appearance. To reach a viscosity of the order of 1000 poises (necessary for its moulding) temperatures are required which, depending on its composition, oscillate between 1220 °C for the richest silicate in SiO2, and 900 °C for the poorest. They are very soluble in water: between 35% and 50% by weight of silicate, depending on the SiO2 content. Their lack of mechanical rigidity and their solubility in water make them useless as a substitute for quartz glass in any of their applications.
They are rarely presented in the industry in solid form, but in the form of an aqueous solution. Its solution in water is used as a highly effective ceramic glue or as a raw material for the production by hydrolysis of silica gel, a substance used as a moisture absorber (gas drying towers, etc.) or as a component of certain products such as vehicle tires and other applications in the chemical industry.
Its production is carried out in continuous balsa furnaces heated by the combustion of petroleum derivatives and frequently also with electricity, at the highest possible temperatures (within a certain profitability) in order to increase the productivity of the furnace. These temperatures are usually between 1400 °C and 1500 °C.
Sodium silicate glasses
In order to obtain a product with properties similar to those of quartz glass at temperatures achievable by technically profitable means, a sodium silicate glass is produced to which other components are added to make it more mechanically resistant, inert to chemical agents at room temperature —very particularly water— and that keep their transparency to light, at least in the visible spectrum.
These components are alkaline earth metals, particularly magnesium, calcium or barium, as well as aluminum and other elements in smaller quantities, some of which appear to be supplied as impurities by the raw materials (in the case of iron, sulfur or others). The raw materials used for the production of glass of this type are chosen among those that present a lower cost:
- For quartz:
- feldspatic sands, purity in SiO2 higher than 95% and with the lowest possible content of ferrous components (between 0.15 % and 0.01 % in terms of Fe2O3)
- Cuaritas molidas
- For the sodium:
- Natural sodium carbonates (United States and Africa foundations).
- Synthetic sodium carbonate, the most used in Europe.
- Synthetic sodium sulfate, by-product of the chemical industry.
- Natural sodium nitrate (natural sodium)nitrate of Chile).
- Sodium chloride or common salt.
- These last three, used in small proportions, due to the detachment of polluting gases during glass processing: SOX, NOXCl2.
- For calcium:
- Natural calibrations.
- For magnesium:
- Natural dolomites.
- For the barium:
- Natural water sulfate (baritin).
- For aluminum:
- Natural Feldespatos (caolines).
The industrial production of this type of glass is carried out, as in the case of sodium silicates, in glass kilns, usually of balsa, heated by combustion of petroleum derivatives with support, in many cases, of electric energy at temperatures ranging between 1450 °C and 1600 °C. In these furnaces a mixture is inserted into powder slightly humid (♥ ♥ {displaystyle sim }5 % of water) and previously dosed of the raw materials already cited. This mixture of mineral matter reacts (at appreciable speeds and, of course, the greater the better) to form the set of silicates that, combined and mixed, will give rise to that substance to which it is called common glass.
Properties of glass
The properties of common glass are a function of both nature and raw materials, as well as the chemical composition of the product obtained. This chemical composition is usually represented in the form of percentages by weight of the most stable oxides at room temperature of each of the chemical elements that form it. The compositions of the most widely used sodium silicate glasses are within the limits established in the attached table.
| Component | From... % | up to % |
| Yes2 | 68.0 | 74,5 |
| Al2O3 | 0.0 | 4.0 |
| Fe2O3 | 0.0 | 0.45 |
| Cao | 9.0 | 14.0 |
| MgO | 0.0 | 4.0 |
| Na2O | 10,0 | 16,0 |
| K2O | 0.0 | 4.0 |
| SO3 | 0.0 | 0.3 |
Many studies—particularly in the first half of the 20th century—have attempted to establish correlations between what was called the internal structure of glass —generally based on atomic theories— and the properties observed in glasses. The product of these studies was a set of relationships, of an absolutely empirical nature, that surprisingly accurately represent many of these properties through linear relationships between the content of the chemical elements that make up a given glass (expressed in the form of percentage content by weight of its most stable oxides) and the magnitude representing said property. Interestingly, correlations with compositions expressed in molar or atomic form are much less reliable.
| Yes2 | Al2O3 | Fe2O3 | Cao | MgO | Na2O | K2O | SO3 |
| 73.20 | 1.51 | 0.10 | 10,62 | 0.03 | 13,22 | 1,12 | 0.20 |
The MgO, Fe2O3 and SO3 contents are a consequence of impurities in limestone, sand and sodium sulfate, respectively.
- Source
- Coefficients for calculating glass properties
| Property | Value | Units | Source |
| Density at 25 °C(1) | 2.49 | g/cm3 | Gilard " Dubrul |
| Linear expansion coefficient at 25 °C(2) | 8,72•10−6 | °C−1 | Wilkelman " Schott " |
| Thermal conductivity at 25 °C | 0.002 | cal/cm.s.°C | Russ |
| Surface tension at 1200 °C | 319 | dynas/cm | Rubenstein |
| Refraction index (to 589.3 nm)(3) | 1.52 | - | Gilard " Dubrul |
| elasticity module at 25 °C | 719 | kbar | Appen |
| Poisson Module at 25 °C | 0.22 | - | Wilkelman " Schott " |
| Traction resistance at 25 °C(4) | ♥ ♥ {displaystyle sim } (900) | bar | Wilkelman " Schott " |
| Dielectric strength (4.5.18)8 Hz) | 7.3 | - | Appen & Bresker |
| Electrical resistance at 1100 °C | 1.06 | ..cm | |
| Electrical resistance at 1500 °C | 0.51 | ..cm | |
| Specific heat to 25 °C | 0.20 | cal/g/°C | Sharp & Ginter |
| Chemistry care DIN 12111(5) | 13,52 | HCl 0.01N | R. Fourth |
- Note
- Viscosity is expressed in Figure 3.
- Source
- Coefficients for the calculation of glass properties (table)
The light absorption (or transparency)(7) of sodium silicate glasses in the visible spectrum (0.40 μ to 0.70 μ) depends on its content in transition elements (Ni and Fe in the example). However, in both ultraviolet and infrared, glass behaves almost like an almost opaque object, regardless of any of these elements.
- Notes
- (1) The density is somewhat higher than in the molten quartz (2.5 vs 2.2 g/cm3).
- (2) The linear thermal dilation coefficient at room temperature is significantly higher than that of the cast silica (about 20 times more), so the sodium silicate glass objects are less resistant to the "thermal layer".
- (3) Its refractive index is slightly higher than that of quartz glass and can be increased by the use of additives.
- (4) The resistance to traction in any type of glass is a magnitude that depends extraordinarily on the condition of the surface of the object in question, so its quantification is complex and unreliable.
- (5) Resistance to chemical or physical attack (dissolution) of common glass is a function of its chemical composition mainly. However, in all of them this resistance is high. It is usually measured by a series of internationally typified tests. Among the most used:
- DIN 12116
- DIN 52322
- DIN 12111
- The acability of glass is also modified by superficial treatments with SO2Sn, Ti, and others.
- (6) A viscosity between 1000 poises and 5000 poises is necessary to mould a glass. In the case of silica temperatures of more than 2600 °C are required, while for common glass only 1 200 °C, approximately.
- (7) The absorption of light is influenced by the intimate structure of these transparent subjects. In the case of a Si-O structure photon absorption is low, even for small wavelengths (transparency to UVA rays). This is not the case when other elements are added to this simple structure (Na, Mg, Ca, etc.) that decisively influence the absorption of small wavelengths (under 200 nm) and infrared (over 700 nm). On the other hand, the presence in the food network transition elements (see Periodic table of the elements) produces selective removals of visible radiation, which allows, among other things, to color the glass with a wide range of shades.
Recycling
Glass recycling is the process through which glass wastes are converted into materials that will serve to create new products.
Glass is a fully recyclable material and there is no limit in the number of times that can be reprocessed. Recycle it does not lose the properties and saves an amount of energy of about 30% regarding the new glass. This recycling allows to reduce the amount of waste that is then taken to the landfill, which means a saving of both raw materials and energy from the manufacture of glass from new raw materials.
In certain cases the glass is reused, rather than recycled. It does not melt, but is used only by washing it (in the case of containers). In glazings, you can also take advantage of the glass by cutting it again (as long as a smaller unit is needed).
In places where glass recycling programs are implemented, special glass containers can be found in public places.Utilities of glass
The main characteristics of glass (its transparency and its hardness), despite the restrictions imposed by its main limitation (its fragility), make it an essential element in numerous applications, forming by itself a group of materials of of enormous economic importance.
Building and architecture
- Cash
Since the middle of the XX century, glass facades have become an almost essential hallmark of large buildings of the main cities in the world. These facades are usually made of flat glass pieces with a wide range of colors, which facilitates the creative work of architects. These glasses are normally subjected to certain processes that improve their thermal and acoustic insulation properties; and its ability to attenuate external light.
In conventional facades, glass continues to maintain its preponderant role in windows, integrated into different types of carpentry (from traditional wood, through steel, aluminum, and even PVC), with single glass or double glass separated by a confined layer of air.
- Interiors
Today, glass has become a primary element in home decoration. Thanks to its elegance, transmission of exterior light and its transparency, glass makes spaces become spacious and clean. For this, the choice of the right glass is very important, especially for architects and designers who are the ones who make use of this material for the creation of their projects.
In addition, as it has different colors and textures, glass can be used in numerous ways in an infinite number of elements, such as:
- Bathroom screens
- Split screens
- Mirrors
- Wall coating
- Barandillas
- Curtains
- Glasses
- Tables
- Lightning
- Glass windows
- Thermal and acoustic insulation
Glass wool is used as thermal and acoustic insulation in buildings, placed between the exterior and interior walls of many buildings.
- Structural elements
There are some pioneering projects that have used fiberglass treated with resins for use in small bridges and walkways, taking advantage of its lightness. Likewise, the use of fiberglass bars for concrete reinforcement has been proposed, thus avoiding the effect of corrosion on metallic reinforcements in especially aggressive environments.
Windshield
Since the first comfortable carriages for the transport of passengers, all the companies that manufacture means of transport (railroads, shipbuilding, the automobile industry and the aerospace industry) have been linked from their origins to the realization of the glass elements used both in windows and windshields as well as in interior and exterior lighting systems of all types of vehicles. Similarly, another element linked to the automobile industry is the manufacture of rear-view mirrors.
A clear example is the evolution of automobile design, which went from exclusively using flat glass to integrating sophisticated curved glass elements in windshields and windows. Both the aerospace and automobile industries have benefited and in turn have made notable contributions to the development of increasingly lighter and stronger glass, such as Gorilla Glass, later widely used in the manufacture of cell phones.
Packaging
Glass (despite competition from cheaper containers such as aluminum or steel cans; bricks of waxed or aluminum-coated cardboard; and plastic bottles) is still one of the containers used preferentially for the commercialization of most alcoholic beverages (among which wine and beer can be included on a massive scale, despite the progression of other types of containers in these two cases), a multitude of preserves (especially jams and vegetables, which benefit from the visibility of the product through glass), soft drinks of all kinds and perfumery products such as colognes or certain beauty products (to which glass containers of original designs provide an undeniable added value).
Since the first half of the XX century, when food companies were in charge of collecting the containers for their cleaning and new use (common practice at that time in dairy, brewing and soft drink industries), until the 1980s, when the use of non-returnable containers destined to be recycled in the manufacture of new bottles, glass has proven to be one of the least polluting materials and one of the easiest to recycle.
Similarly, the pharmaceutical industry frequently uses glass containers for many of its liquid preparations such as syrups or injectables.
Energy production
Energy production systems such as photovoltaic panels and solar thermal power plants massively use glass elements to capture solar energy. In the case of photovoltaic panels, they protect the silicon cells (and eventually concentrate the light), and in the case of solar thermal power plants, they are the key element of the collecting mirrors (and in some systems, also of the collectors through which they circulate). the fluids with which the sun's heat accumulates).
The improvement of the properties of these glasses (cost, transparency, thermal and chemical stability, resistance to dirt and environmental agents...) is key to the profitability of the expensive investments necessary for the commissioning of these glasses facilities.
Optics
It constitutes one of the main specific applications of glass since the Renaissance, when quality lenses began to be produced with increasingly sophisticated procedures. Some of the scientific foundations of optics had already been laid before (since the year 1000 Arab mathematicians such as Alhacen had studied the geometry of mirrors). However, it was not until Galileo Galilei appeared with his lens telescope, Anton van Leeuwenhoek with his primitive microscope, and Isaac Newton himself with the development of the mirror telescope, when the foundations for the importance of optical instruments were definitively established., reaching the theoretical limits of resolution at the beginning of the 20th century, with Carl Zeiss's realizations based on the discoveries Ernst Abbe's theories, based on the use of different types of glass.
The applications of glass optical technology are mainly focused on the instruments for the treatment and capture of images; in scientific devices for the study of light; in digital communications; and in the ophthalmological correction of human vision defects through lenses:
- Image capture
- Telescopes
- Cameras
- Film chambers
- TV cameras
- Video cameras
- Topographic and air photography equipment
- Earth observation satellites
- Prismatics
- Periscopes
- Microscopes
- Reproduction of images
- Projectors
- Outreach equipment
- Photocopiers
- Communications
- Optical fibre
- Computer
- Optical readers and recorders (CD, DVD, Blue Ray)
- Scientific instrument
- Spectrometers
- Interferometers
- Laser-ray equipment
- Educational material
- Planetary
- Ophthalmology
- Gafas
- Optometry equipment
Laboratory material
A large part of the equipment in chemical and pharmaceutical laboratories (test tubes, beakers, flasks, pipettes, condensers, slides for microscope slides...) are made of glass. Sometimes special glasses are used, prepared to withstand high temperatures or certain chemical attacks.
Appliances
Televisions systematically use glass screens to protect the different systems of luminous pixels through which they form the images.
Conventional ovens, microwave ovens and ceramic hobs include heat-resistant glass elements in their design.
Similarly, washing machines usually incorporate a circular glass door, and many refrigerators use glass shelves to improve the feeling of space and interior light.
Lighting
Since the invention of gas or oil flame lamps, glass bell jars have been used to prevent both flame extinguishment and accidental propagation. With the invention of the electric incandescent light bulb, the characteristic glass bulb that protects the filament has become an irreplaceable element, which has been progressively adapted to the higher thermal requirements demanded by sodium vapor lamps, halogens (with pure silica glasses) or xenon (using special glasses). Even in fluorescent tubes, whose operating temperatures are low, the glass that contains the neon gas is an essential element. Only the development of LED light systems (due to their low heat emission) can allow glass to be replaced by translucent plastic materials that are cheaper, lighter and easier to manufacture.
Many models of lamps in which light points are mounted use glass elements to disperse and give a certain decorative aspect to the light they project. In this sense, we can mention the enormous chandeliers made up of numerous pieces of crimped glass, characteristic of the great halls of public and private buildings from the Victorian Era to the First World War.
Cell phones and touch devices
The use of luminous screens (increasingly larger) has become widespread in cell phones and touch devices, made of particularly resistant glass, such as Gorilla Glass.
Watchmaking
Traditionally, watch faces have been protected with domed glass, later adopting flat profiles. In the case of wristwatches, it is an essential requirement both when they mount needle devices (to prevent them from being damaged) and when it comes to digital devices (the glass allows the screen to be shown to the outside). High-end watches usually mount sapphire crystal, whose extraordinary hardness prevents them from being easily scratched.
Kitchen and household items
Many kitchen utensils can be made of glass (such as serving dishes or bowls). The advent of borosilicate glass, capable of withstanding very high temperatures, dramatically expanded the use of glass in the kitchen, to the point of turning it into a material widely used in dishes for preparing roasts in the oven.
On the table, both the glasses and all kinds of cups, as well as jugs and containers for most liquids, are usually made of glass, and there is also tableware in which the plates are also made of this material, replacing to ceramics.
Decoration and jewelry
Special quality colored glass is frequently used in costume jewellery, replacing much more expensive natural gems. An example is Swarovski crystal, which is used to produce a wide range of decorative products, as well as fashion accessories linked to costume jewellery.
In other types of products, beads and colored glass beads are frequently sold and are used to make handmade necklaces and bracelets, both for marketing and for pure entertainment.
Main manufacturing companies
According to the data published by the website CIAdvanced, the main glass manufacturing industries in the world (the vast majority share this activity with the manufacture of ceramic materials), are concentrated in Japan, Europe and the United States.
The economic potential of these companies is considerable. The largest of them, the French Saint-Gobain, had a turnover of 45,000 million dollars in 2014 and employed nearly 60,000 workers, with some 3,700 dedicated to research and development tasks. Its main customers are the automotive industry, aerospace, power plants and ophthalmic optics. The size of the companies in Japan is also notable, which concentrates four of the top ten.
The smallest of the ten, the Austrian RHI AG, in 2014 had a turnover of 1.9 billion dollars, and employed 8,000 workers.
On the other hand, the American company Owens-Illinois is the world's largest manufacturer of glass containers. It invoices 7.4 billion dollars annually and employs 24,000 workers. (See: Glass manufacturing)
Contenido relacionado
Deuterium
Cogeneration
Esterification