Fermium
Fermium is an artificially created radioactive chemical element whose atomic number is 100 and atomic weight 254, symbol Fm. There are 16 known isotopes with 257Fm being the most stable with a half-life of 100.5 days. Fermium is one of the transuranic elements of the actinide group of the periodic system. The element was isolated in 1952, from the remains of a hydrogen bomb explosion, by the American chemist Albert Ghiorso and his colleagues. Fermium was later synthetically prepared in a nuclear reactor by bombarding plutonium with neutrons, and in a cyclotron by bombarding uranium 238 with nitrogen ions. Isotopes with mass numbers from 242 to 259 have been obtained; fermium 257, which has the longest life, has a half-life of 80 days. The element was given the name fermium in 1955, after the Italian-American nuclear physicist Enrico Fermi. Fermium has no industrial applications.
Discovery
Fermium was discovered in the products precipitated to the ground from the 'Ivy Mike' (1 November 1952), the first successful hydrogen bomb test. Initial examinations of the explosion residue had shown the production of a new isotope of plutonium, Pu, Z=94, A=244: it could only be formed by the absorption of six neutrons by a uranium-238 nucleus followed by two β decays. At the time, the absorption of neutrons by a heavy nucleus was believed to be an uncommon and rare process, but the identification of Pu-244 raised the possibility that uranium nuclei could absorb even more neutrons, giving rise to new elements.
Element 99 (einsteinium) was quickly discovered on filter papers that had been blown through the explosion cloud (the same sampling technique that had been used to discover Pu-244). It was later identified in December 1952 by Albert Ghiorso and his collaborators at the University of California at Berkeley. They discovered the isotope 253Es (half-life 20.5 days) which was formed by the capture of 15 neutrons per the uranium-238 nucleus, which then underwent seven successive beta decays:
[+ 15 {ce {n}}][7 beta^-] ^{253}_{99}Es}}}" xmlns="http://www.w3.org/1998/Math/MathML">U92238→7β β − − +15nThat's it.99253{displaystyle {ce {^{238}_{92}U - 2005[+ 15 {ce {n}}}[7 beta^-] ^{253}_{99}It's}}}}}
[+ 15 {ce {n}}][7 beta^-] ^{253}_{99}Es}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/38f0bd3ea3e7092c2e8d58a9cb7b20044a2680e3" style="vertical-align: -2.391ex; margin-top: -0.357ex; margin-bottom: -0.614ex; width:15.973ex; height:6.343ex;"/>
(1)
However, some 238U atoms were able to capture a different number of neutrons (more likely 16 or 17).
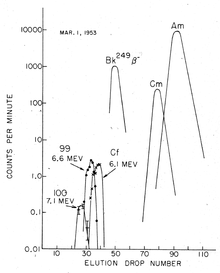
The discovery of fermium (Z = 100) required more material, as the yield was expected to be at least an order of magnitude lower than that of element 99, the contaminated coral of the Atoll of Enewetak (where the test was performed) was sent to the University of California Radiation Laboratory in Berkeley, California for processing and analysis. About two months after the test, a new element emitting high-energy α particles (7.1 MeV) with a half-life of about 1 day was isolated. With such a short half-life, it could only arise from the β− decay of an isotope of einsteinium, and therefore it must be an isotope of the new element 100: it was quickly identified as 255Fm (t = 20.07(7) hours).
The discovery of the new elements, as well as the new data on neutron capture, was initially kept secret by order of the US military until 1955 due to Cold War tensions. However, the team at Berkeley managed to prepare elements 99 and 100 by civilian means, by neutron bombardment of plutonium-239, and published this work in 1954 with the caveat that they were not the first studies that had been done on the elements. 1955 the "Ivy Mike" studies were declassified and published.
The Berkeley team was concerned that another group might discover lighter isotopes of element 100 using ion bombardment techniques before they could publish their classified research, which they did. A group at the Nobel Institute for Physics in Stockholm independently discovered the element, producing an isotope later confirmed to be 250Fm (t1/2 = 30 minutes) by bombarding a target of U-238 atoms with oxygen-16 ions, and published their work in May 1954. However, the Berkeley team's priority was generally recognized, and with it the prerogative of naming the new element in honor of the recently deceased Enrico Fermi, the developer of the first self-sustaining artificial nuclear reactor.
Features
Fermium is not found in nature; its discovery and production is achieved by artificial transmutation of lighter elements. Radioactive isotopes of mass number 244-259 have been discovered. The total mass of the fermium that has been synthesized is much less than a millionth of a gram.
Spontaneous fission is the primary mode of decay for 244Fm, 256Fm, and 258Fm. The longest-lived isotope is 257Fm, which has a half-life of about 100 days. Fermium -258 decays by spontaneous fission and has a half-life of 0.38 milliseconds. This suggests the existence of an abnormality at this point in the periodic table.
Production
Fermium is produced by bombarding lighter actinides with neutrons in a nuclear reactor. Fermium-257 is the heaviest isotope made by neutron capture, and can only be produced in picogram quantities. The main source is the 85 MW High Flux Isotope Reactor (HFIR) at Oak Ridge National Laboratory in Tennessee, United States, which is dedicated to the production of transcurium (Z > 96) elements. The lower mass fermium isotopes are available in larger quantities, although these (254Fm and 255Fm) are comparatively short-lived. In a "typical processing campaign" at Oak Ridge, tens of grams of curium are irradiated to produce decigrams of californium, milligrams of berkelium and einsteinium, and picograms of fermium. However, nanogram quantities of fermium are prepared for specific experiments. Quantities of fermium produced in thermonuclear explosions of 20–200 kilotons are believed to be on the order of milligrams, although it is found mixed with large amounts of waste; 4.0 picograms of 257Fm were recovered from 10 kg of waste from the "Hutch" (July 16, 1969). The Hutch experiment produced an estimated total of 250 micrograms of 257Fm.
After being produced, the fermium must be separated from the other actinides and the lanthanide fission products. This is typically done using ion exchange chromatography, where the standard process uses a cation exchanger such as Dowex 50 or TEVA eluted with ammonium α-hydroxysobutyrate solution. Smaller cations they form more stable complexes with the α-hydroxysobutyrate anion, and are preferentially elucidated by the column. A rapid fractional crystallization method has been described.
Although the most stable isotope of fermium is 257Fm, with a half-life of 100.5 days, most studies are done with 255Fm (t1/2 = 20.07(7) hours), since this isotope can be easily isolated as needed since the decay product of 255 It is (t1/2 = 39.8(12) days).
Chemistry
The chemistry of fermium has only been studied in solution using techniques for minute quantities, and no solid compounds have been prepared. Under normal conditions, fermium exists in solution as the ion Fm3+, which has a hydration number of 16.9 and an acid dissociation constant of 1.6×10−4 (pKa = 3.8). Fm3+ forms complexes with a wide variety of ligands with strong donor atoms such as oxygen, and these complexes are generally more stable than those of the preceding actinides. It also forms anionic complexes with ligands such as chloride or nitrate, and again these complexes appear to be more stable than those formed with einsteinium or californium. The binding in the latter actinide complexes is thought to be mainly ionic in character: the Fm3+ ion is thought to be smaller than the preceding An3+ ions due to of the higher effective nuclear charge of fermium and therefore fermium would be expected to form shorter and stronger metal bonds.
Fermium(III) can be easily reduced to fermium(II), for example with samarium(II) chloride, with which fermium(II) co-precipitates. In the precipitate, the compound is produced fermium(II) chloride (FmCl2), although it has not been purified or studied in isolation. The electrode potential has been estimated to be similar to that of the ytterbium(III) pair. /(II), or about −1.15 V relative to the standard hydrogen electrode, a value in agreement with theoretical calculations. The pair Fm2+/Fm0 has an electrode potential of −2.37(10) V based on polarographic measurements.
Contenido relacionado
Uracil
Ligand
Methane