Fermentation
The fermentation or fermentative metabolism is a catabolic process of incomplete oxidation, which does not require oxygen, and whose final product is an organic compound. It is characteristic of the metabolism of many microorganisms and depending on the final products, there are various types of fermentation. The science that studies fermentation is zymology.
Wine is a sea of organisms. Some people live, others are broken.Louis Pasteur.
It was discovered by Louis Pasteur, who described it as "la vie sans l'air" (life without air). Typical fermentation is carried out by yeasts. Also some metazoans, protists and various prokaryotic organisms are capable of performing it.
The fermentation process is anaerobic, that is, it occurs in the absence of oxygen; this means that the final acceptor of the electrons of the NADH produced in glycolysis is not oxygen, but an organic compound that will be reduced in order to be able to reoxidize the NADH to NAD+. The organic compound that is reduced (acetaldehyde, pyruvate...) is a derivative of the substrate that has been previously oxidized.
In living beings, fermentation is an anaerobic process and does not involve mitochondria or the respiratory chain. The fermentation process is characteristic of some microorganisms: some bacteria and yeast. It is also produced in most animal cells (including humans), except in neurons, which die rapidly if they cannot perform cellular respiration. Some cells, such as red blood cells, lack mitochondria and are forced to ferment; Animal muscle tissue undergoes lactic fermentation when oxygen supply to muscle cells is not sufficient for aerobic metabolism and muscle contraction.
From an energetic point of view, fermentations are very unprofitable when compared to aerobic respiration, since only two molecules of adenosine triphosphate (ATP) are obtained from one glucose molecule, while in the aerobic respiration occurs from 36 to 38. This is due to the oxidation of NADH which, instead of penetrating the respiratory chain, gives up its electrons to organic compounds with little oxidizing power.
In industry, fermentation can be oxidative, that is, in the presence of oxygen, but it is an incomplete aerobic oxidation, such as the production of acetic acid from ethanol.
Biochemical Summary
The fermentation is a metabolic process that generally converts carbohydrates into acids, gases or alcohols to extract part of the chemical energy from them while reoxidizing the coenzymes reduced by these reactions. It is a redox metabolic pathway in which the ultimate electron acceptor is often confused with the final product of the reactions. It is characterized by a partial degradation of the fermentable substance and allows only limited energy production. It takes place in yeasts and bacteria as well as in muscle cells that lack oxygen, that is, in anaerobic conditions. Its characterization in the 19th century contributed to the discovery of enzymes. Louis Pasteur thus estimated that ferments were responsible for alcoholic fermentation in yeast.
More precisely, fermentation is a mode of cellular respiration that implements an electron transfer system based on small soluble molecules from the cytosol —often organic acids or their derivatives— and not on a membrane respiratory chain. Its ATP production often occurs by phosphorylation at the substrate level as opposed to oxidative phosphorylation and is significantly lower than the latter. ATP production by fermentation, on the other hand, is faster than by oxidative phosphorylation because it takes place in the cellular compartment where ATP is consumed, without the need to go through an ATP/ADP translocase.
The first stage common to all modes of fermentation is glycolysis, which converts glucose to pyruvate with phosphorylation of two ADP molecules to ATP and reduction of two NAD+ molecules to NADH:
C6H12O6 + 2 NAD+ + 2 ADP + 2 Pi → 2 ATP + 2 NADH + 4 H+ + 2 H2O + 2 CH3COCOO−
ATP is used in cellular processes that require energy, such as biosynthesis, active transport across membranes, and cell motility. Rather, NADH must be oxidized back to NAD+ to allow cellular metabolism to continue. The main function of fermentation is to ensure this reoxidation, transferring electrons from NADH to an electron acceptor which is then removed from the cell. During lactic fermentation, for example, the electron acceptor is pyruvate itself, which is then reduced to lactate: this is what happens in the muscles during intense physical effort, which exceeds the cellular oxygenation capacities and causes glycolysis to occur. of the cytosol works much faster than the respiratory chain of the mitochondria.
Etymology
The word “fermento” derives from the Latin verb fervere, which means to boil. Fermentation would thus come from the Late Latin fermentatio, -ōnis It is believed to have been used for the first time at the end of the century XIV in alchemy, but only in a broad sense. It was not used in the modern scientific sense until around 1600.[citation needed]
Definitions
Here are some definitions of fermentation. They range from general and informal uses to more scientific definitions.
- Food conservation methods through microorganisms (general use).
- Any large-scale microbial process that occurs with or without air (common definition used in industry).
- Any process that produces alcoholic beverages or acid dairy products (general use).
- Any metabolic process of energy release that takes place only in anaerobic conditions (something scientific).
- Any metabolic process that releases energy from a sugar or other organic molecule, which does not require oxygen or an electron transport system, and uses an organic molecule as a final electron acceptor (more scientific).
History of the use of fermentation
Fermentation is a natural phenomenon that occurs during the decomposition of organic matter and that men were able to experience when they saw how the fruits fermented without their intervention.
The conscious use of fermentation by humans dates back to the Paleolithic for food preservation and the Neolithic for the production of certain beverages: it has been documented to date from 7000 to 6600 BC. C. in Jiahu, China, 5000 BCE. C. in India (Ayurveda mentions many medicinal wines), 6600 B.C. C. in Georgia, 3150 B.C. C. in ancient Egypt, 3000 B.C. C. in Babylon, 2000 a. C. in pre-Hispanic Mexico, and 1500 B.C. in Sudan. Fermented foods have religious significance in Judaism and Christianity. The Baltic god Rugutis was worshiped as the agent of fermentation.
Fermentation has also been used for the detoxification of wild or domesticated plants (for example, lattice fermentation of cassava, lactic fermentation of wild cabbages to make sauerkraut, fermentation of toxic legumes such as soybeans, miso, néré seeds), the increase in the bioavailability of minerals (for example, the baking of cereals rich in demineralizing phytates - barley, millet, starch, engrain, spelt, wheat - thanks to the phytase of the yeasts that eliminate these phytates during fermentation), the production of dairy products whose fermentation eliminates toxic lactose from milk (typically cheeses that eliminate it in the whey and by lactic fermentation in the curd poorly tolerated by adult populations of the time or whose lactase in ferments retains its activity (characteristic of yogurts). Fermentation thus allows Neolithic men to adapt to the nutritional transition due to to the new food resources introduced by agriculture and livestock, a transition marked by the generalization of the consumption of cereals and cultivated legumes, and by the appearance of the consumption of fermented dairy products.
Canning developed in the XIX century, ending traditional meat preservation techniques such as salting, smoking, but also systematic tenderizing of venison.
At the beginning of the 21st century there are more than 5000 foods in the world that tradition owes to fermentation (alcoholic or non-alcoholic beverages, dairy products, meat products, such as salami, or vegetables such as sauerkraut).
Scientific Understanding
In 1837, Charles Cagniard de la Tour, Theodor Schwann, and Friedrich Traugott Kützing independently published papers that concluded, as a result of their microscopic investigations, that yeast was a living organism that reproduced by budding. Schwann boiled grape juice to kill the yeast and found that no fermentation occurred until new yeast was added. However, many chemists, including Antoine Lavoisier, continued to view fermentation as a simple chemical reaction and rejected the notion that living organisms could be involved. This was seen as a reversion to vitalism and was satirized in an anonymous publication by Justus von Liebig and Friedrich Wöhler.
The turning point came when Louis Pasteur (1822-1895), during the 1850s and 1860s, repeated Schwann's experiments and showed that fermentation was initiated by living organisms in a series of investigations. In In 1857, Pasteur showed that lactic acid fermentation was caused by living organisms. In 1860, he demonstrated how bacteria cause milk to sour, a process previously thought to be simply a chemical change. His work in identifying the role of microorganisms in food spoilage led to the pasteurization process.
In 1877, working to improve the French brewing industry, Pasteur published his famous article on fermentation, "Etudes sur la Bière", which was translated into English in 1879 as "Studies on fermentation". He defined fermentation (incorrectly) as "Life Without Air", however, it correctly showed how specific types of microorganisms caused specific types of fermentations and end products.[citation needed]
Although showing that fermentation was the result of the action of living microorganisms was a breakthrough, it did not explain the basic nature of fermentation; Nor did it show that it was caused by microorganisms that seemed to be always present. Many scientists, including Pasteur, had tried unsuccessfully to extract the fermentation enzyme from yeast.
Success came in 1897 when the German chemist Eduard Buchner ground up yeast, extracted a juice from it, and then discovered to his amazement that this "dead" liquid could ferment a sugar solution, forming carbon dioxide and alcohol, very similar to what live yeasts did.
Buechner's results are considered to mark the birth of biochemistry. The "disorganized ferments" behaved just like the organized ones. From that moment on, the term enzyme came to be applied to all ferments. It was then that it was understood that fermentation was caused by enzymes produced by microorganisms.In 1907, Buechner won the Nobel Prize in Chemistry for his work.
Advances in microbiology and fermentation technology have continued steadily to the present. For example, in the 1930s, it was discovered that microorganisms could be mutated by physical and chemical treatments so that they had higher yields, grew faster, tolerated less oxygen, and could use a more concentrated medium. Selection was also developed. of strains and hybridization, which affected most modern food fermentations.[citation needed]
Types of fermentation
There are several types of fermentation, according to which aspect is considered. The simplest is the one that distinguishes between natural fermentation, when environmental conditions allow the interaction of microorganisms and susceptible organic substrates, and artificial fermentation, when human beings favor conditions and the referred contact.
They are also classified according to the nature of the products of the fermentation reactions, the best known being the four that start from pyruvate: alcoholic, lactic, butyric and acetic.
Other types of fermentation are:
- butyric fermentation II
- butanodialic fermentation, a variant of lactic fermentation, carried out by enterobacteria that release carbon dioxide and generate butanodiol, a colorless and viscous alcohol.
- fermentation cocoa production
- hydrogenic fermentation
- kójica fermentation
- malolactic fermentation
- Propionic fermentation.
It can also be complex or combined processes, as in the following cases:
- fermentation ABE (aceto-butylic-ethyl)
- Mixed acid fermentation, which mainly affects enterobacteria, that is, the bacteria of the digestive tract.
- fermentation of synthesis gas
Biochemical description
Alcoholic fermentation
Alcoholic fermentation is carried out in particular by yeasts and converts carbohydrates such as glucose, fructose and sucrose —a disaccharide formed from the two preceding— into ethanol CH
3CH
2OH and carbon dioxide CO
2 with production of a small amount of metabolic energy in the form of ATP.
During the formation of ethanol (reaction 2 below), pyruvate CH
3COCOO– resulting from glycolysis (reaction 1) is first decarboxylated to acetaldehyde CH
3CHO with release of one carbon dioxide molecule CO
2, then [reduced in ethanol CH
3CH
2OH by alcohol dehydrogenase with oxidation of a NADH molecule to NAD+:
(1)C6H12O6 + 2 NAD+ + 2 ADP + 2 Pi → 2 ATP + 2 NADH + 4 H+ + 2 H2O + 2 CH3COCOO−
(2)CH3COCOO– + NADH + 2 H+ → NAD+ + CH3CH2OH + CO2
(1+2)C6H12O6 + 2 ADP + 2 Pi → 2 ATP + 2 H2O + 2 CH3CH2OH + 2 CO2
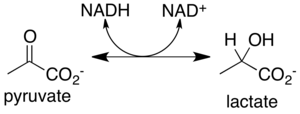
Lactic acid fermentation
Lactic acid fermentation is a metabolic pathway, produced by certain bacteria and some animal cells, which convert carbohydrates such as glucose, other hexoses and disaccharides formed by hexoses into lactate CH
3CHOHCOO– with production of a small amount of metabolic energy in the form of ATP.
During lactate formation (reaction 2 below), pyruvate CH
3COCOO– resulting from glycolysis (reaction 1) is reduced to lactate by lactate dehydrogenase with oxidation of a NADH molecule to NAD+:
(1)C6H12O6 + 2 NAD+ + 2 ADP + 2 Pi → 2 ATP + 2 NADH + 4 H+ + 2 H2O + 2 CH3COCOO−
(2)CH3COCOO– + NADH + H+ → NAD+ + CH3CHOHCOO–
(1+2)C6H12O6 + 2 ADP + 2 Pi → 2 ATP + 2 CH3CHOHCOO – + 2 H+ + 2 H2O
For comparison, in the presence of dioxygen, respiration produces up to 36-38 moles of ATP from one mole of glucose, which is about 18-19 times more than fermentation. It mobilizes a more complex enzymatic apparatus (see Krebs cycle and respiratory chain). In evolutionary terms, fermentation is favored whenever there are large amounts of sugar and little oxygen, which corresponds to the conditions of life before the appearance of oxygen in the Earth's atmosphere. As soon as sugar becomes scarce and/or oxygen becomes abundant, as it started about two billion years ago and ended about 250 million years ago, respiration occurs and organisms, specialists capable of implementing it. Note that mitochondria, the site of cellular respiration, are organelles descended from α-proteobacteria.
Malolactic fermentation
It is carried out by bacteria. Allows stabilizing wines for aging.
The corresponding chemical equation is the following (transformation of malic acid into lactic acid):
HOOCCH2CHOHCOOH → CH3CHOHCOOH + CO2
Acetic fermentation or acetification
Principle of acetic fermentation or acetification
"Acetic fermentation" (improper) or acetification is a redox reaction of carbohydrates, primary alcohols, polyols or aldehydes in acetic acid resulting from acetic bacteria.
The chemical equation for ethanol fermentation is:
CH3CH2OH + O2 → CH3COOH + H2O + 348 kJ
Accessibility process
In an aqueous solution of ethanol (such as an alcoholic beverage) or unfermented carbohydrates (such as a must), acetic fermentation produced by acetic bacteria contributes to volatile acidity, particularly those resulting directly from alcoholic fermentation in the one with carbohydrates: wine, beer, sake, cider, perry, mead...
Beyond a critical rate of volatile acidity, acetification is at the origin of an acescence, a colony of acetic bacteria (mainly of the genera Acetobacter aceti, Gluconoacetobacter europaeus, Gluconobacter oxydans and Acetobacter orleanensis) organize themselves into a biofilm during this process: the mother of vinegar. Furthermore, Gluconobacter oxydans can produce polysaccharides (glucan, lavender...) during maturation, thus making the medium viscous.
Beyond a specific critical level of acetic acid in volatile acidity, an aqueous ethanol solution can undergo acetic pitting by esterification of acetic acid and ethanol to ethyl acetate.
Since the oxidative catabolism of acetic bacteria is aerobic, acetic fermentation does not qualify as a fermentation in the strict sense, their cellular respiration arises from a membrane respiratory chain.
Physiological roles
Alcoholic fermentation
Alcoholic fermentation or ethylic fermentation is carried out by many living organisms (bacteria, yeasts) permanently or occasionally in environments devoid of oxygen. The property of certain yeasts to transform sugar into ethanol is used by man in the production of alcoholic beverages (and not alcoholic beverages, as can be read incorrectly in the press or heard, because alcoholization occurs spontaneously and not through the addition of ethanol/alcohol) and for the manufacture of bread. The ideal temperature for fermentation is 35°C to 40°C.
Alcoholic beverages are obtained by natural fermentation of sugar solutions (musts). It is a natural chemical (biochemical) reaction obtained thanks to microorganisms (bacteria, molds, fungi) and yeasts that, thanks to their enzyme, zymase, break down the natural fruit juices into ethanol and carbon dioxide bubbles..
Yeasts are naturally present on the surface of fruits or added to musts (fruit juice) that are fermented. Specifically, to trigger the fermentation process, all you have to do is leave the fruit in contact with the air, taking care to crush the biological protection membranes (skin, etc.), which is done by crushing or crushing the fruit. The yeasts suspended in the air are more than enough to produce the fermentation of the porridge in a few days.
Yeasts can also be added to speed up this natural process, such as brewer's (or bread's) yeast as well, keeping the temperature around 37 °C, fermentation occurs in about an hour.
This phenomenon has been known scientifically from the work of the chemists Jean-Antoine Chaptal (following the work of François Rozier and Antoine Lavoisier), Gay-Lussac (1817), Pasteur (1866) and Buchner (1897) who demonstrated the enzymatic nature of the transformation of sugar into ethanol. His knowledge is related to chemistry, enzymology and microbiology.
Lactic acid fermentation
Lactose fermentation
Lactic acid fermentation is widely used in cheese making. Yoghurts are obtained from boiled milk, then cooled and inoculated with a defined strain of bacteria, for example L. Bulgaricus (Lactobacillus delbrueckii subsp. bulgaricus), and incubated according to the fermentation process and the product to be fermented.
Fermentation of cabbage in sauerkraut
Sauerkraut is made by lactic fermentation in the presence of 2 to 3% sodium chloride. The process is stopped when the lactic acid content reaches approximately 1.5%.
Silage (agriculture)
Lactic acid fermentation is favored during silage of agricultural products, because the acidity produced prevents the development of other microorganisms that can cause putrefaction of silage products.
Fermentation by intestinal flora
The presence of lactic ferments in the intestinal flora is very favorable for a proper functioning of the intestine[citation required].
Muscle contraction and lactic fermentation
Finally, during the anaerobic processes that govern muscle contraction, glycogen, which is a glycosylated polymer, releases glucose thanks to an enzyme, glycogen phosphorylase, glucose then joins glycolysis and forms two equivalents of pyruvate. These are then converted to lactic acid by lactate dehydrogenase, which is subsequently oxidized during aerobic processes. Lactic acid fermentation is a chemical reaction that can take place when oxygen deprivation occurs in muscle cells: muscles need a large amount of energy during physical activity, and they consume a large amount of sugar and, above all, oxygen. The glucose and oxygen necessary for the cellular respiration reaction are stored in the cell and renewed by the bloodstream. The amount of oxygen supplied may not be sufficient, either in the event of a short and intense effort (taking into account the time between the flow at rest and the flow at full effort), or even when the maximum oxygen flow is already reached (during the final sprint), while sugar remains available; the muscle cells then carry out lactic fermentation to produce energy.
Increased concentration of lactate ions in muscle cells is one of the reasons for fatigue after intense activity. In fact, these lactate ions change the intracellular pH and in fact modify the enzymatic operating conditions of the cell so that it can no longer function properly.
However, recent research suggests that increased K+ ions may be to blame, while excess lactate (ionized form of lactic acid) results in increased muscle performance[citation needed]. Excess lactate is "recycled" into pyruvate by liver cells.
Acetic fermentation in vinegar factory
Some species of acetic bacteria form colonies that are involved in vinegar making in the acescence of aqueous solutions containing ethyl alcohol. They are organized in a biofilm called mother of vinegar, in the presence of air in a container that is not ouillé (act of substituting the wine evaporated in the barrel with a wine of the same origin).
Colonies of acetic bacteria that are highly tolerant to ethanol and acetic acid are also involved in the acescence of an aqueous solution of ethyl alcohol resulting from the alcoholic fermentation of a must: molasses for spirit vinegar, grape must for wine, malt for beer, apple wort for cider, pear wort for perry, honey wort for mead, rice wort for rice vinegar, banana Cavendish wort for banana beer...
The species of acetic bacteria involved in the acescence process in wine vinegar are mainly: Acetobacter aceti, Gluconobacter oxydans, Acetobacter orleanensis, Acetobacter œni and Gluconoacetobacter europaeus whose tolerance to ethanol and acid acetic is the highest. Deposited on a surface of 1 m², a colony of the latter constitutes a biofilm of 0.5 g/ m2 (dry state) in 24 hours at 20 °C. Within 48 hours, a single bacterium produces its own weight of acetic acid, provided it has a large amount of oxygen or a hydrogen acceptor (particularly methylene blue). Its rationalized cultivation allows accelerating the process of acescence that previously required at least three weeks.
Gluconoacetobacter kombuchae, Acetobacter aceti, Gluconobacter oxydans and Gluconoacetobacter xylinum can develop in symbiosis with certain yeasts (schizosaccharomyces pombe, Brettanomyces bruxellensis, Torulaspora delbrueckii…) in some drinks similar to vinegar, especially in kombucha which is an acid drink that has undergone acetic fermentation...
Acetobacter pomorum is mainly involved in the production of cider and perry vinegars, Acetobacter lambici in beer vinegar, Acetobacter papayae in those tropical fruit vinegars whose must underwent alcoholic fermentation: pineapple vinegar, banana vinegar Cavendish, passion fruit, soursop, mango papaya...
Fermentation processes
Winemaking
In winemaking, it is the yeasts found naturally during flowering that, after pressing (white and rosé wines) or during fermentation (red wines), will transform the sugar present in the berries into alcohol. the grape.
In oenology, the main objectives of the alcoholic fermentation of grape must into wine are the following:
- ensure complete and rapid fermentation of sugars;
- avoid the production of volatile acidity during fermentation;
- avoid the production of sulfur compounds with unpleasant odors during fermentation;
- achieve the aromatic and enjoyable goal.
These stages can be favored by adding a yeast selected for its fermentative characteristics.
Carrying out the alcoholic fermentation of a grape must requires controlling the factors that directly affect the life and survival of a yeast population.
Beer fermentation
Fermentation is one of the stages in the production of beer. This step consists of inoculating the must with a certain amount of yeast so that these yeasts convert the sugars present into alcohol and CO
2. There are four types of fermentation: low, high, spontaneous and mixed.
Faisandage
Deer meat ripening is due to bacterial fermentation of its intestinal contents, proteases from bacteria that disorganize the robust muscles and tendons (proteolysis process) developed by wildlife hunting.
Metanization
In the field of organic waste treatment and renewable energy production, methanation (anaerobic digestion) allows organic matter (organic pollution, manure, fermentable domestic waste) to be transformed into biogas. It mainly consists of four phases:
- polymer hydrolysis of sugars, proteins or lipids in monomers;
- acidogenesis that allows the transformation of these monomers into volatile fatty acids;
- acetogenesis that produces acetate;
- methanegenesis for the production of methane and CO
2.
Applications
The primary industrial benefit of fermentation is the conversion of wort into wine, barley into beer, and carbohydrates into carbon dioxide to make bread. Other uses of fermentation are the production of supplements such as cyanocobalamin, etc.
According to Steinkraus (1995), the fermentation of food serves 5 general purposes:
- Enrichment of the diet through the development of a variety of flavors, aromas and textures in the food substrates.
- Preservation of substantial amounts of food through lactic acid, ethanol, acetic acid and alkaline fermentations.
- Enrichment of dietary substrates with protein, amino acids, essential fatty acids and vitamins.
- Detoxification during the food fermentation process.
- Decrease in cooking times and fuel requirements.
Fermentation has some unique uses for food. It can produce important nutrients or remove antinutrients. Food can be preserved by fermentation, fermentation uses energy from food and can create unsuitable conditions for undesirable organisms. For example, vinegaring the acid produced by the dominant bacterium inhibits the growth of all other microorganisms. Yogurt and kefir are also obtained by fermentation of milk.
Depending on the type of fermentation, some products (eg fusel alcohol) can be harmful to health. In alchemy, fermentation is often the same as putrefaction, meaning to allow the natural rotting or decomposition of the substance.
Contenido relacionado
Konrad Lorenz
Anapsid
Rutherford's atomic model