Exocytosis

1. Mitochondria,
2. Synaptic vesicle with neurotransmitters,
3. Self-receptor,
4. Synapsis with released neurotransmitter (serotonin),
5. Post-anaptic receptors activated by neurotransmitters (induction of post-septic potential),
6. Calcium channel,
7. Exocytosis of a vesicle and
8. Recaptured neurotransmitter
Exocytosis (from the Greek ἔξω exo: outside and κύτος cyto: receptacle) is a form of active transport and transport by entrainment in which a cell transports molecules (for example, neurotransmitters and proteins) out of the cell (exo- + cytosis). As an active transport mechanism, exocytosis requires the use of energy to transport material. All cells use exocytosis and its counterpart, endocytosis, because most of the substances important to them are large polar molecules, which cannot cross the hydrophobic portion of the cell membrane by passive transport. Exocytosis is the process by which a large number of different molecules are released at once, and is called bulk cargo. Exocytosis occurs through portals in the cell plasma membrane called porosomes, which are a cup-shaped lipoprotein structure in the plasma membrane, where secretory vesicles dock and transiently fuse to release their intravesicular contents.
In exocytosis, membrane-bound secretory vesicles are transported to the cell membrane, where they dock and fuse into porosomes and their contents (ie, water-soluble molecules) are secreted into the extracellular environment. This secretion is possible because the vesicle is transiently fused with the plasma membrane. In the context of neurotransmission, neurotransmitters are normally released from synaptic vesicles into the synaptic cleft through exocytosis; however, neurotransmitters can also be released by reverse transport through membrane transport proteins.
Exocytosis is also a mechanism by which cells can insert membrane proteins (such as ion channels and cell surface receptors), lipids, and other components into the cell membrane. The vesicles containing these membrane components fuse completely and become part of the outer cell membrane.
History
The term was proposed by Christian de Duve in 1963.
Types
In eukaryotes there are two types of exocytosis: one is Ca2+-triggered non-constitutive exocytosis (i.e., regulated exocytosis) and the other type is non-Ca2+-triggered constitutive exocytosis. triggered by Ca2+ (ie, unregulated). Vesicular exocytosis in gram-negative prokaryotic bacteria is a third mechanism and the last finding in exocytosis.
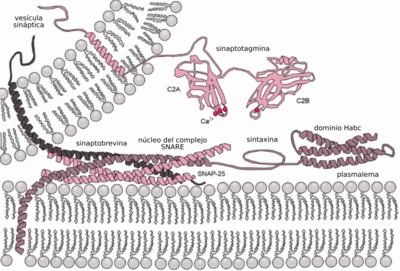
Ca2+-triggered exocytosis non-constitutive requires an external signal, a specific sorting signal in the vesicles, a clathrin coating, as well as an increase in intracellular calcium. In multicellular organisms, this mechanism initiates many forms of intercellular communication, such as synaptic transmission, hormone secretion by neuroendocrine cells, and immune cell secretion. In neurons and endocrine cells, SNARE proteins and SM proteins catalyze fusion by forming a complex that bridges the two fusion membranes. For example, in synapses, the SNARE complex is formed by syntaxin-1 and SNAP25 on the plasma membrane and VAMP2 on the gallbladder membrane. Exocytosis at neuronal chemical synapses is triggered by Ca2+ and serves for interneuronal signaling. Calcium sensors that trigger exocytosis could interact with the SNARE complex or with the phospholipids of the fusion membranes. Synaptotagmin has been recognized as the major sensor for Ca2+-triggered exocytosis in animals. However, synaptotagmin proteins are absent in plants and unicellular eukaryotes. Other possible calcium sensors for exocytosis are EF hand proteins (eg calmodulin) and C2 domain (eg Ferlins, E-synaptotagmin, Doc2b) containing proteins. It is not clear how the different calcium sensors can cooperate together and mediate the kinetics of calcium-triggered exocytosis in a specific way.
Constitutive exocytosis is performed by all cells and serves for the release of extracellular matrix components or the supply of newly synthesized membrane proteins that are incorporated into the plasma membrane after fusion of the transport vesicle. There is no clear consensus on the machinery and molecular processes that drive the formation, budding, translocation, and fusion of post-Golgi vesicles to the plasma membrane. Fusion involves membrane anchoring (recognition) and membrane fusion. It is not yet clear if the machinery between constitutive and regulated secretion is different. The machinery required for constitutive exocytosis has not been studied as much as the mechanism of regulated exocytosis. Two anchoring complexes are associated with constitutive exocytosis in mammals, ELKS and Exocyst. ELKS is a large spirally coiled protein, also involved in synaptic exocytosis, marking the fusion points of 'hot spots' in synapses. from the fusion of secretory transporters. The exocyst is an octameric protein complex. In mammals, exocyst components are localized to both the plasma membrane and the Golgi apparatus, and exocyst proteins co-localize at the fusion point of post-Golgi vesicles. The membrane fusion of constitutive exocytosis is probably mediated by SNAP29 and Syntaxin19 on the plasma membrane and YKT6 or VAMP3 on the vesicle membrane.
Vesicular exocytosis occurs in gram-negative prokaryotic bacteria. The periplasm is pinched off like bacterial outer membrane vesicles (OMVs) to translocate microbial biochemical signals into eukaryotic host cells or other microbes located nearby, achieving control of the secretory microbe in its environment, including host invasion, endotoxemia, competing with other microbes by nutrition, etc. This finding of membrane vesicle trafficking occurring at the host-pathogen interface also dispels the myth that exocytosis is a purely eukaryotic cell phenomenon.
Steps
Five steps are involved in exocytosis:
Vesicle transit
Certain vesicle transit steps require the transport of a vesicle over a moderately short distance. For example, vesicles that transport proteins from the Golgi apparatus to the cell surface area are likely to use motor proteins and a cytoskeletal cue to approach their target. Before tethering would have been appropriate, many of the proteins used for active transport would have been configured for passive transport, because the Golgi apparatus does not require ATP to transport proteins. Both the actin base and the microtubule base are involved in these processes, along with several motor proteins. Once the vesicles reach their targets, they come into contact with anchoring factors that can restrict them.
Anchoring vesicles
It is useful to distinguish between the initial loose attachment of the vesicles to their target from more stable packaging interactions. Docking involves binding at distances of more than half the diameter of a vesicle from a given membrane surface (>25 nm). Docking interactions are likely involved in the concentration of synaptic vesicles at the synapse.
Vesicle docking
The secretory vesicles transiently dock and fuse at the porosome of the cell plasma membrane, through a tight t-/v-SNARE ring complex.
Priming vesicles
In neuronal exocytosis, the term priming has been used to include all molecular rearrangements and ATP-dependent modifications of proteins and lipids that occur after the initial attachment of a synaptic vesicle, but prior to exocytosis, so the influx of calcium ions is all that is necessary to trigger the almost instantaneous release of neurotransmitters. In other cell types, whose secretion is constitutive (ie, continuous, calcium ion independent, untriggered) there is no priming.
Vesicle Fusion
Transient vesicle fusion is driven by SNARE proteins, resulting in the release of vesicle contents into the extracellular space (or in the case of neurons into the synaptic cleft).
Fusion of the donor and acceptor membranes accomplishes three tasks:
- The surface of the plasma membrane increases (by the surface of the merged vesicle). This is important for cell size regulation, for example, during cell growth.
- Substances within the gallbladder are released outside. These may be waste or toxin products, or signaling molecules such as hormones or neurotransmitters during synaptic transmission.
- Proteins embedded in the vesicle membrane are now part of the plasma membrane. The side of the protein that looked toward the interior now look to the exterior from the cell. This mechanism is important for the regulation of transmembrane and conveyors.
Vesicle Recovery
The recovery of synaptic vesicles occurs by endocytosis. Most synaptic vesicles are recycled without complete membrane fusion (kiss-and-run fusion) through the porosome. Non-constitutive exocytosis and subsequent endocytosis are energy-intensive processes and therefore dependent on mitochondria.
Examination of cells after secretion using electron microscopy demonstrates a greater presence of partially empty vesicles after secretion. This suggested that during the secretory process, only a part of the vesicular content can leave the cell. This could only be possible if the vesicle temporarily established continuity with the cell plasma membrane in the porosomes, expelled a portion of its contents, then detached, resealed, and withdrew into the cytosol (endocytosed). In this way, the secretory vesicle could be reused for subsequent rounds of exoendocytosis, until it is completely empty of its contents.
Contenido relacionado
Alphaherpesvirinae
Echinaria capitata
Dichelachne