Ether (chemistry)
In organic chemistry and biochemistry, an ether is a functional group of the type R-O-R', where R and R' They are alkyl groups, the same or different, with the oxygen atom attached to them.
An ether can be obtained from the condensation reaction between two alcohols (although it is not usually produced directly and intermediate steps are used):
- ROH + HOR' → ROR' + H2O
Usually the alkoxide, RO-, of alcohol ROH is used, obtained by reacting alcohol with a strong base. The alkoxide can react with any compound R'X, where X is a good leaving group, such as iodide or bromide. R'X can also be obtained from an alcohol R'OH.
- RORO- + R'X → ROR' + X-
They do not form hydrogen bonds as they do not have a hydrogen attached to oxygen, and due to this they present a high hydrophobicity, and do not tend to be hydrolyzed. Ethers are often used as organic solvents.
They are usually quite stable, do not react easily, and it is difficult for the carbon-oxygen bond to be broken. Normally, to break it, a strong acid such as hydroiodic acid is used, heating, obtaining two halides, or an alcohol and a halide. An exception are oxiranes (or epoxides), where the ether is part of a highly stressed three-atom cycle, so it easily reacts in different ways.
The bond between the oxygen atom and the two carbons is formed from the corresponding sp³ hybrid orbitals. Two nonbonding pairs of electrons remain on the oxygen atom.
The two pairs of non-bonding electrons of oxygen can interact with other atoms, thus the ethers acting as ligands, forming complexes. An important example is that of crown ethers, which cannot selectively interact with cations of alkaline elements or, to a lesser extent, alkaline earth elements.
Diethyl ether
The term "ether" is also used to refer only to the ether called "diethyl ether, diethyl ether" (according to the IUPAC in its 1993 recommendations "ethoxyethane") or sulfuric ether, with the chemical formula CH3CH< sub>2OCH2CH3. It was isolated and discovered by the alchemist Raymundus Lullis in 1275. It was first synthesized by Valerius Cordus in 1540. It was first used as an anesthetic by Crawford Williamson Long on March 30, 1842.
Crown ethers
They are those molecules that have several ethers in their structure and that also form a cycle are called crown ethers. In the name of the crown ether, the first number refers to the number of atoms that make up the cycle, and the second number, to the number of oxygens in the cycle. Other related compounds are cryptates, which contain, in addition to oxygen atoms, nitrogen atoms. Cryptates and crown ethers are often referred to as 'ionophores'.
These compounds have the oxygen atoms oriented towards the inside of the cycle, and the alkyl chains towards the outside of the cycle, being able to complex cations inside. The importance of this fact is that these compounds are capable of solubilizing insoluble salts in nonpolar solvents. Depending on the size and identity of the crown, it may have a greater or lesser affinity for a certain cation. For example, 12-crown-4 has a high affinity for the lithium cation, 15-crown-5 for the sodium cation, and 18-crown-6 for the potassium cation.
In biological organisms, they usually serve as transport for alkaline cations so that they can pass through cell membranes and thus maintain optimal concentrations on both sides. For this reason they can be used as antibiotics, such as valinomycin, although certain crown ethers, such as 18-crown-6, are considered toxic, precisely because of their excessive affinity for potassium cations and because they unbalance their concentration in cell membranes.
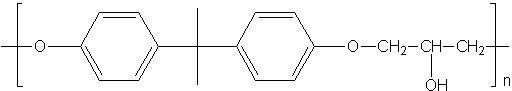
Polyethers
Polymers containing the ether functional group can be formed. An example of formation of these polymers:
- R-OH + n(CH)2)O → R-O-CH2-CH2-O-CH2-CH2-O-CH2-CH2-O...
The best-known polyethers are epoxy resins, which are mainly used as adhesives. They are prepared from an epoxide and a dialcohol.
The epoxides or oxiranes are ethers in which the oxygen atom is one of the atoms in a cycle of three. They are therefore heterocyclic compounds. The cycles of three are highly stressed, so they react easily in opening reactions, both with bases and with acids.
Nomenclature
- The nomenclature of the ethers according to the 1993 recommendations of IUPAC (currently in force) specify that these compounds belonging to the oxygenated functional group should be named as AlcoxialcansI mean, like they're substitutes. The Eter functional group should be specified as a lower priority compared to most organic chains. Each radical ether will be accompanied by suffix oxi.
- A simple compound, such as CH3-O-C6H5 according to the rules of the IUPAC would be called:
- metoxibencen
- The traditional or classic nomenclature (also accepted by the IUPAC and valid for simple ethers) specifies that the substitutes or alkylic remains of the organic chain on the left side of the word ether should be named in alphabetical order. The previous compound would be called according to old rules (already disused) in this way:
- fenil methyl ether
Aliphatic or linear chain simple ethers can be named by adding the suffix -yl to the end of the word ether after the prefixes met-, et-, but-, etc., as indicated the number of carbons. An illustrative example would be the following:
For more details, see Nomenclature of Ethers and Epoxides
Synthesis of ethers
- Williamson's ether synthesis is the most reliable and versatile ether synthesis. This method involves an SN2 attack from an alcoxide ion to a primary alkyl halure not prevented or tosialate. Side alkylo halures and tosialates are occasionally used in Williamson's synthesis, but there is competition in elimination reactions, so performances are often low.
- Alcoxide is usually obtained by adding Na, K or NaOH to alcohol.
- Synthesis of ethers by alcoxymercury-demercury. In the process of alcoxymercuriation-demercuriation, an alcohol molecule is added to a double link of an alcheno. An ether is obtained as shown below:
- Industrial synthesis: bimolecular dehydration of alcohols.
- Reaction of Arens-van Dorp
Reactions of ethers
Rupture by HBr and HI
Ethers react with HBr and concentrated HI, since these reagents are acidic enough to protonate ether, and bromide and iodide ions are good nucleophiles for substitution. A protonated ether can undergo substitution or elimination, with the release of an alcohol.
Self oxidation
Uses of ethers
- Means to extract to concentrate acetic acid and other acids.
- A means of trawling for the dehydration of ethyl and isopropylic alcohols.
- Disolvent organic substances (oils, fats, resins, nitrocellulose, perfumes and alkaloids).
- Initial diesel engine fuel.
- Strong glues.
- Abdominal anti-inflammatory for after delivery, exclusively for external use.
- Poison for rats.
Contenido relacionado
Polonium
Sodium chloride
Food additive