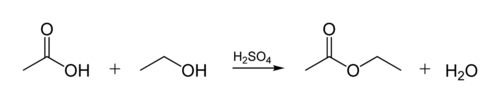
Esterification
The process by which an ester is synthesized is called esterification. An ester is a compound formally derived from the chemical reaction between a carboxylic acid and an alcohol.
When speaking of esters, reference is made to esters of carboxylic acids, substances whose structure is R-COOR', where R and R' They are alkyl groups. However, esters of virtually all inorganic oxyacids can be formed in principle. For example, carbonic esters derive from carbonic acid and phosphoric esters, of great importance in biochemistry, derive from phosphoric acid.
| Steady. (carboxylic acid) | Coal oil (carbonic acid sester) | Phosphoric ester (phosphoric acid) |
|---|---|---|
 | 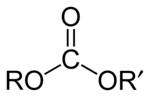 | Archive:Photoshopester.PNG |
Production of esters
The chemical industry produces large amounts of esters. Of particular importance are glycerol esters, ethyl acetate, butyl acetate, dibutyl phthalate, cellulose acetate, cellulose xanthogenate, glyceryl trinitrate, vinyl acetate and cellulose nitrate.
Methyl salicylate is an ester that is used primarily as a flavoring agent and has the advantage of being absorbed through the skin. Once absorbed, methyl salicylate can be hydrolyzed to salicylic acid, which acts as an analgesic. This product can be extracted from various medicinal plants.
Acetylsalicylic acid or aspirin, as it is commercially known, is the most popular of synthetic medications, due to its analgesic, antipyretic, anti-inflammatory and antirheumatic action, and because it is used in the prevention and treatment of acute heart attack. myocardium, in addition to its low cost.
Benzocaine or ethyl p-aminobenzoate is a local anesthetic, used as a pain reliever, also obtained by esterification.
Plastics such as polyethylene terephthalate (PET) is an example of a polymer obtained by an esterification process.
Structure Chemistry
Given the importance of esters, many processes have been developed to obtain esters. The most common is heating a mixture of the alcohol and the corresponding acid in the presence of catalytic amounts of sulfuric acid, using the cheaper reagent in excess to increase the yield of the reaction (Fischer-Speier esterification). Sulfuric acid serves in this case both as a catalyst and as a hygroscopic substance that absorbs the water formed in the reaction (it is sometimes replaced by concentrated phosphoric acid). In general, this procedure requires high temperatures and long reaction times, thus presenting drawbacks; The alcohol can undergo elimination reactions forming, esterification with sulfuric acid itself or the formation of the corresponding symmetrical ether. Similarly, the organic acid to be esterified can undergo decarboxylation.
For this reason, more active derivatives of the acid are often used. In the synthesis of acetylsalicylic acid, for example (the ester between the hydroxyl group of salicylic acid and acetic acid), one starts from acetic anhydride and salicylic acid. A molecule of acetic acid is released that can be easily separated from the product:
O(OCCH3)2 + HO(C)6H4)COOH - rigid CH3COOH + H3CCOO(C)6H4)COOH
Another process is the use of acid chloride (R-COCl) (Einhorn's variant) and alcohol in the presence of pyridine. In this case, hydrochloric acid is released which reacts directly with the pyridine in the medium to give pyridine hydrochloride. The conditions of this reaction are very mild since it does not require the presence of strong acids or bases and it can be carried out at room temperature or even lower. For this reason, it allows the synthesis of compounds in the presence of very sensitive functional groups.
Transesterification processes are also used where an ester is reacted with an alcohol in the presence of a catalyst such as germanium tetrachloride, another Lewis acid or traces of base. The previously bound alcohol is released in the form of the ester. This process is used industrially, especially in obtaining PET (polyethylene terephthalate), a transparent plastic that is used, for example, to manufacture drink bottles.
There are still processes of minor importance such as the addition of an acid to an olefin, etc., which in the same way form esters.
Chemical mechanism
The esterification reaction goes through a nucleophilic attack of oxygen from an alcohol molecule to the carbon of the carboxylic group. The proton migrates to the hydroxyl group of the acid which is then removed as water. The role of the catalyst is to increase the carbonyl activity (the partial positive charge on the carbon atom) by protonation of one of the acid's oxygens. The same can be achieved by using more active derivatives of the acid such as halides or anhydrides.
Properties of esters
They are usually colorless and hydrophobic substances (they do not mix with water). Low molecular weight esters usually have a characteristic odor. Many natural plant flavors are esters, and synthetic ones are used as artificial flavors (isoamyl acetate has a banana flavor, while allyl hexanoate has a pineapple flavor).
Esters have solvent properties and are often used as such (ethyl acetate).
Contenido relacionado
Aspartame
Nicotine
Palmitic acid