Essential chemical element
essential chemical elements refer to a series of elements that are considered essential for life or for the subsistence of certain organisms. For an item to be considered essential, it must meet four conditions:
- Insufficient intake of the element causes functional deficiencies, reversible if the element is again at appropriate concentrations.
- Without the element, the organism does not grow or complete its life cycle.
- The element directly influences the organism and is involved in its metabolic processes.
- The effect of that element cannot be replaced by any other element.
Classification
Most are light atoms or light elements. They are generally classified according to their abundance in macroelements, trace elements and ultratrace elements. Trace and ultra-trace elements are called trace elements. The following list shows the bioelements present in humans ordered according to their abundance.
- Macroelements or abundant elements: oxygen, carbon, hydrogen, nitrogen, calcium, phosphorus, potassium, sulphur, sodium, chlorine, iron, magnesium.
- Elements Trace (see Oligoelements): fluoride, zinc, copper, silicon, vanadio, tin, selenium, manganese, iodine, nickel, molybdenum, chromium, cobalt.
- Ultratrace elements: They are those elements that are required in a dose less than 1 mg per day. The essentiality of such elements is not demonstrated, except for iodine and molybdenum.
Not all living beings have the same proportion of essential elements, for example tungsten is an essential chemical element for some living beings. The following periodic table highlights the recognized essential elements, as well as some that could be considered, such as lithium, cadmium, arsenic and tin.
Periodic table and essential elements
H | He | |||||||||||||||||||||||||||||||
Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||
Na | Mg | Al | Yeah. | P | S | Cl | Ar | |||||||||||||||||||||||||
K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Separate | Br | Kr | |||||||||||||||
Rb | Mr. | And | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | You | I | Xe | |||||||||||||||
Cs | Ba | La* | Hf | Ta | W | Re | You | Go | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |||||||||||||||
Fr | Ra | Ac* | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||||||||||||||
| Element majority | Element trace | Essentiality discussed |
Causes of essentiality
There are elements that are in an organism, but are believed to be non-essential. In the event that one wants to check whether the deficiency of an element can affect an organism, the study is complicated by the small concentrations that are handled: it is possible that the element reaches the organism inadvertently or it can happen that the organism is able to endure with the reserves it has and deficiency is not observed until several generations have passed. Essentiality is usually demonstrated when a biological function is discovered for some compound of the element. These chemical elements are believed to have become essential due to their abundance and affordability. Thus, there is a good relationship between the essentiality of an element and its abundance in the earth's crust or in seawater.
In cases where an element is abundant but not essential, it is explained taking into account that it is difficult to dispose of. For example, aluminum is a very abundant element in the earth's crust and it is not an essential element, probably because it forms highly insoluble compounds in water and cannot be easily taken up by organisms. Conditions have also changed since the beginning of life and organisms have been able to adapt to the changes produced. For example, iron is now inaccessible, as it is mainly as Fe3+ which forms poorly soluble compounds and organisms have to form soluble complexes to uptake it. However, when the atmosphere was less oxidizing it was found mainly as Fe2+, which does form more soluble compounds.
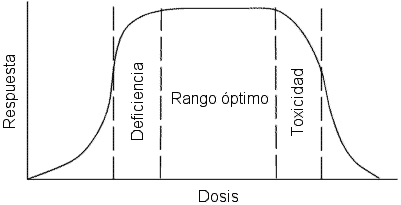
Dose-response relationship
Any element, whether essential or not, can be toxic above certain concentrations. For each essential element there is a range of concentrations considered optimal for an organism. In this range, a concentration is reached with which the functions that depend on that element can be developed correctly, but it is not excessively high as to produce toxic effects.
Below this range, deficiency occurs in this element, which leads to the appearance of pathological effects or even the death of the organism.
Above the optimal range there are also pathological effects or death of the organism derived from the toxicity of the element.
In an organism, optimal levels of an element are maintained by "homeostatic mechanisms." In this way the absorption, storage and excretion of the elements is controlled. However, a deficit or excess can occur due to diet, problems in the absorption mechanisms, etc...
Contenido relacionado
Come to
Transverse muscle of the nose
(66) Pestle