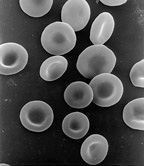
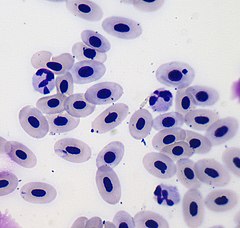
Erythrocyte
The erythrocytes (from the Greek ἐρυθρός 'red', and κύτος 'bag') also called red blood cells or red blood cells, are the cells more numerous than blood. Hemoglobin is one of its main components, and its function is to transport oxygen to the different types of tissues in the body. Human erythrocytes, as well as those of other mammals, lack a nucleus and mitochondria, so they must obtain their metabolic energy through lactic fermentation. The amount considered normal in humans fluctuates between 4,500,000 (in women) and 5,400,000 (in men) per cubic millimeter (or microliter) of blood, that is, approximately 1000 times more than leukocytes. Excess red blood cells are called polycythemia and deficiency is called anemia. Red blood cells are commonly used in transfusions in clinical practice and have been suggested as carriers for drugs and nanoparticles. The life time is 120 days, later it dies by a process called apoptosis.
Description
The erythrocyte is a biconcave disk 5–7.5 μm in diameter, 1 μm thick, and 80–100 femtoliters in volume. The cell has lost its residual RNA and its mitochondria, as well as some important enzymes; therefore, it is unable to synthesize new proteins or lipids. Their cytoplasm mostly contains the pigment hemoglobin, which gives them their characteristic red color (which can be darker depending on their oxygenation) and is responsible for oxygen transport.
Now, this description applies to mammalian erythrocytes, since in the rest of vertebrates, with some exceptions, erythrocytes lack the biconcave shape and are usually larger than those described above. This is because the red blood cells of the rest of the vertebrates still have a nucleus.
Red blood cells are derived from committed stem cells called blood stem cells. Erythropoietin, a growth hormone produced in renal tissues, stimulates erythropoiesis (ie, the formation of red blood cells) and is responsible for maintaining a steady state red blood cell mass. Erythrocytes, like leukocytes, originate from the bone marrow.
Red cell concentrations vary by gender, age, and geographic location. Higher erythrocyte concentrations are found at high altitude, in males, and in newborns. Decreases below the reference range lead to a disease state called anemia. This alteration causes tissue hypoxia. Increased red blood cell concentration (polycythemia) is less common.
Hemolysis is the destruction of aged erythrocytes and occurs in macrophages of the spleen and liver. The essential elements, globin and iron, are preserved and reused. The heme fraction of the molecule is catabolized to bilirubin and biliverdin, and is ultimately excreted through the intestinal tract. Intravascular rupture of the erythrocyte releases hemoglobin directly into the blood, where the molecule dissociates into α and β dimers, which bind to the transport protein, haptoglobin. This transports the dimers to the liver, where they are subsequently catabolized to bilirubin and excreted.
Red Blood Cells in Mammals
Mammalian erythrocytes do not have a nucleus when they reach maturity, that is, they lose their cell nucleus and therefore their DNA; amphibians, reptiles, and birds have nucleated erythrocytes. Red blood cells also lose their mitochondria and use glucose for energy through the process of glycolysis followed by lactic fermentation.
Red blood cells are continuously produced in the bone marrow of long bones, although in the embryo, the liver is the main producer of red blood cells. The spleen acts as a reservoir for erythrocytes, but its role is somewhat limited in humans. However, in other mammals, such as dogs and horses, the spleen releases large numbers of red blood cells during times of stress. Some athletes have tried to exploit this function of the spleen by trying to release its erythrocyte reserves through drugs, but this practice puts the cardiovascular system at risk, since it is not prepared to support blood whose viscosity is higher than what is considered normal.
Human RBCs
Red blood cells have a flattened, biconcave, oval shape with a depression in the center. This design is optimal for the exchange of oxygen with the surrounding medium, as it gives them flexibility to be able to cross the capillaries, where they release the oxygen load. The diameter of a typical erythrocyte is 6-8 µm. Red blood cells contain hemoglobin, which is responsible for transporting oxygen and carbon dioxide. It is also the pigment that gives blood its red color.
Values considered normal for erythrocytes in adults
- Women: 4.7 ± 0.7 x 106/μL (microliter) blood
- Men: 5.3 ± 0.8 x 106/μL (microliter) blood
Red blood cell maturation
Given the constant need to replenish red blood cells, the erythropoietic cells in the bone marrow are among the fastest growing and reproducing cells in the entire body. Therefore, as might be expected, its maturation and production are greatly affected in cases of significant nutritional deficiencies.
For the final maturation of erythrocytes, two vitamins are needed in particular: vitamin B12 and folic acid. Both are essential for DNA synthesis because both, in different ways, are required for the formation of thymidine triphosphate, one of the essential components of DNA. Therefore, the lack of vitamin B12 or folic acid causes a decrease in the production of DNA and, consequently, determines a failure of maturation and nuclear division.
Also, the erythroblastic cells of the bone marrow, in addition to not proliferating rapidly, give rise mainly to larger than normal red blood cells called macrocytes, with a very thin, irregular, and oval membrane, instead of the usual biconcave disc. These malformed cells, after entering the circulating blood, carry oxygen normally, but due to their fragility, their life is shortened by half to one third. For this reason, it is said that a deficiency of vitamin B12 or folic acid produces a failure of erythropoietic maturation.
There are other causes that alter red blood cell maturation, such as iron deficiency and other genetic abnormalities that lead to the production of abnormal hemoglobins. All these problems will lead to alterations of the erythrocytes, by alteration of the membrane, the cytoskeleton or others.
Stages of morphological development
The stages of morphological development of the erythroid cell include (in order of increasing maturity) the following stages:
- Multipotential mother cell.
- Multipotential mother cell.
- Parental cell or CFU-S (training unit of spleen colonies).
- BFU-E (erythrocyte shoot forming unit).
- CFU-E (forming unit of erythrocyte colonies), which will then form proeritroblasts.
- Proeritroblasto: Large cytoplasm cell abundant, large chromatin nucleus thick, not very well defined nucles (20-25 microns).
- Basophilic erythroblast: Smaller than the previous (16-18 microns), basophilic cytoplasm, thick chromatin and grumosa, here the formation of hemoglobin begins.
- Eritroblasto policromatófilo: It measures 10-12 microns, cytoplasm begins to acquire a pink color by the presence of hemoglobin, here is the last mitotic phase for the formation of hematis, it does not possess nucléolos and the core/cytoplasm ratio is 4:1.
- Orthochromatic erythroblast: It measures 8-10 microns, has compact chromatin and the nucleus begins to disappear.
- Reticulocito: Almost differentiated in mature erythrocytes. The presence of reticulocytes in peripheral blood indicates the good functioning of the bone marrow.
- Eritrocito, finally, when it already lacks nucleus and mitochondria. It has transport capacity (gases, hormones, medicine, etc.).
As the cell matures, hemoglobin production increases, causing a change in the color of the cytoplasm in blood samples stained with Wright's stain, from dark blue to reddish-gray and pinkish. The nucleus gradually becomes pyknotic, and is ejected out of the cell in the orthochromatic stage.
The erythrocyte membrane is a bilipid–protein complex, which is important in maintaining cell deformability and selective permeability. As the cell ages, the membrane becomes rigid, permeable, and the erythrocyte is destroyed in the spleen. The average half-life of the normal erythrocyte is 100 to 120 days.
Membrane composition
The erythrocyte membrane has several roles that help regulate surface deformation, flexibility, adhesion to other cells, and immune recognition. These functions are highly dependent on their composition, which defines their properties. The erythrocyte membrane is composed of three layers: the glycocalyx on the outside, which is rich in carbohydrates; the lipid bilayer that contains several transmembrane proteins in addition to their major lipid constituents; and the membrane cytoskeleton, a structural network of proteins located on the inner surface of the lipid bilayer. Half of the erythrocyte membrane mass in humans and most mammals is protein, the other half is lipid, mainly phospholipid and cholesterol.
Membrane lipids
The red blood cell membrane is composed of a lipid bilayer, similar to that found in virtually all human cells. This lipid bilayer is composed of cholesterol and phospholipids in equal proportions by weight. The lipid composition is important because it defines many physical properties such as permeability and fluidity. Furthermore, the activity of various membrane proteins is regulated by their interaction with the lipid bilayer. Unlike cholesterol, which is evenly distributed between the inner and outer monolayers, the 5 main phospholipids are arranged asymmetrically:
In the outer monolayer
- Fosfatidilcolina
- Sfingomylin
In the inner monolayer
- Fosfatidiletanolamina
- Fosfoinositol
- Fosfatidilserina
The asymmetric distribution of phospholipids in the bilayer is the result of the function of some energy-dependent and energy-independent phospholipid transport proteins. Flippases are proteins that move phospholipids from the outer to the inner monolayer, while the so-called flopases do the reverse operation, against the concentration gradient in an energy-dependent manner. In addition, there are the scramblase proteins that move phospholipids in both directions at the same time, due to their concentration and energy-independent gradients. The identity of the membrane maintenance proteins in erythrocytes is still under discussion.
The maintenance of the asymmetric distribution of phospholipids in the bilayer is critical for the integrity and functionality of the cell due to several reasons:
- The macrophages recognize and empower erythrocytes that have exposed phosphatidylserin on the external surface. Therefore keeping phosphatidylserin in the inner monolayer is essential for the survival of the cell in its frequent encounters with macrophages of the reticulous-endothelial system, especially in the spleen.
- Premature destruction of thalassemic and falsiform erythrocytes have been linked to the disorganization of lipidic asymmetry leading to the exposure of phosphatidylserin in the outer monolayer.
- A phosphatidylserin exposure can enhance the adherence of erythrocytes to vascular endothelial cells, effectively avoiding normal transit through microvasculatura. It is therefore important to keep phosphatidylserin in the inner flap of the bicapa to ensure a normal blood flow in micro-circulation.
- Phosphatidylserin and phosphatidilinositol-4,5-biphosphate (PtdIns(4,5)P2) may regulate the mechanical function of the membrane, due to its interactions with cytoeskeletal proteins such as spectrine and protein 4.1R. Recent studies point out that the union of spectrine to phosphatidylserine promotes mechanical stability in the membrane. The PtdIns(4,5)P2 improves the binding of the 4.1R protein to glycoforin C but decreases its interaction with the 3 protein band, and thus can modulate the bonding of the bicapa to the cytoskeleton.
The presence of specialized structures called lipid rafts in the erythrocyte membrane have been described in recent studies. These cholesterol-rich and sphingolipid-rich structures are associated with specific membrane proteins, such as the G protein.
Energy metabolism of the erythrocyte
The metabolism of erythrocytes is limited, due to the absence of a nucleus, mitochondria, and other subcellular organelles. Although the binding, transport, and release of oxygen and carbon dioxide is a passive process that does not require energy, there are a variety of energy-dependent metabolic processes that are essential for cell viability.
The most important metabolic pathways for the mature erythrocyte require glucose as a substrate. These pathways refer to:
- glucolysis
- path of the pentose phosphate
- via the hemoglobin reductasa
- Rapoport-Luebering cycle
These pathways contribute energy by maintaining:
- high intracellular potassium, low intracellular sodium and very low intracellular calcium (cation pump);
- reduced hemoglobin;
- high levels of reduced glutathione;
- integrity and deformability of the membrane.
Embden–Meyerhof pathway or anaerobic glycolysis
Provides ATP for the regulation of the intracellular concentration of cations (Na+, K+, Ca2+, Mg 2+) through cation pumps. The erythrocyte obtains energy in the form of ATP from the breakdown of glucose by this pathway. Normal erythrocytes do not have glycogen stores, they are completely dependent on environmental glucose for glycolysis. Glucose enters the cell by facilitated diffusion, a process that does not consume energy. It is metabolized to lactate, where it produces a net gain of two moles of ATP for one mole of glucose.
Pentose cycle
Provides nicotinamide-adenine dinucleotide phosphate and reduced glutathione to reduce cellular oxidants. Approximately 5% of cellular glucose enters the pentose oxidative pathway, a helper system for producing reduced coenzymes. Reduced glutathione protects the cell against many injuries caused by permanent oxidizing agents. Oxidants within the cell oxidize the sulfhydryl (-SH) groups of hemoglobin, unless the oxidants are reduced by reduced glutathione. This is why the function of this pathway is crucial in the erythrocyte.
Hemoglobin reductase pathway
Protects hemoglobin from oxidation via NADH and methemoglobin reductase. This is an alternate pathway to the Embden–Meyerhof pathway, essential for maintaining heme iron in the reduced Fe++ state. Hemoglobin with iron in the ferric state, Fe3+, is known as methemoglobin. This form of hemoglobin fails to combine with oxygen. Methemoglobin reductase, in conjunction with NADH produced by the Embden–Meyerhof pathway, protects heme iron from oxidation. Without this system, the 2% methemoglobin formed each day would rise to 20-40% over time, severely limiting the oxygen-carrying capacity of the blood. Oxidizing drugs can interfere with methemoglobin reductase and cause even higher methemoglobin values. This causes cyanosis.
Rapoport–Luebering Cycle
This cycle is part of the Embden–Meyerhof pathway, and its purpose is to prevent the formation of 3–phosphoglycerate and ATP. BPG (2,3-bisphosphoglycerate) is present in the erythrocyte at a concentration of one mol BPG/mol hemoglobin, and it binds strongly to deoxyhemoglobin, thereby maintaining hemoglobin in a deoxygenated state and facilitating release of oxygen. The increased concentration of diphosphoglycerate facilitates the release of oxygen to the tissues by decreasing the affinity of hemoglobin for oxygen. In this way, the erythrocyte has an internal mechanism for regulating the supply of oxygen to the tissues.
Hemoglobin
It is a special pigment that gives red blood cells their characteristic red color. Its molecule has iron, and its function is the transport of oxygen. It is present in all animals except some groups of lower animals. It participates in the process by which the blood carries the necessary nutrients to the body's cells and carries its waste products to the excretory organs. It also carries oxygen from the lungs (or from the gills, in fish), where it is taken up by the blood, to the tissues of the body.
When hemoglobin binds with oxygen to be carried to the body's organs, it is called oxyhemoglobin. When hemoglobin binds to CO2 to be eliminated by expiration, which occurs in the lungs, it is called carboaminohemoglobin (hemoglobin is also called deoxyhemoglobin when it is not bound to oxygen). If hemoglobin binds to carbon monoxide (CO), then a very stable compound called carboxyhemoglobin is formed, which has a very strong bond with the heme group of hemoglobin and prevents oxygen uptake, thus easily generating a anoxia leading to death.
Hemoglobin also carries waste products and carbon dioxide back to the tissues. Less than 2% of the total oxygen, and most of the CO2, are held in solution in the blood plasma. Hemoglobin represents 35% of the weight of the erythrocyte. A related compound, myoglobin, acts as an oxygen store in muscle cells.
Contenido relacionado
Mimosoideae
Lythraceae
Jet lagged