Ernest Rutherford
Ernest Rutherford, also known as Lord Rutherford (United Kingdom: /'ɜːnɪst 'rʌðəfəd/; Brightwater, August 30, 1871-Cambridge, October 19, 1937), was a New Zealand physicist.
He dedicated himself to the study of radioactive particles and managed to classify them into alpha (α), beta (β) and gamma (γ). He found that radioactivity was accompanied by a disintegration of the elements, which earned him the Nobel Prize in Chemistry in 1908. An atomic model is owed to him, with which he proved the existence of the atomic nucleus, in which all the positive charge and almost all the mass of the atom. He achieved the first artificial transmutation with the collaboration of his disciple Frederick Soddy (Soddy later in his career also received the Nobel Prize in Chemistry in 1921 for his work on isotopes).
During the first part of his life he devoted himself entirely to research, spending the second half teaching and directing the Cavendish Laboratories in Cambridge, where the neutron was discovered. He was a teacher, among others, of Niels Bohr and Otto Hahn.
Early Years
Her father, James, of Scottish origin, was a farmer and mechanic, and her mother, English-born Martha Thompson, was a teacher, who had emigrated before she was married. Both wanted to give their children a good education and try to enable them to continue their studies.
Rutherford was early noted for his curiosity and his ability for arithmetic. His parents and his teacher encouraged him a lot, and he turned out to be a brilliant student, which allowed him to enter Nelson College, where he spent three years. He also had great qualities for rugby, which made him very popular at his school. The last year, he finished first in all subjects, thanks to which he entered the University in New Zealand, at Canterbury College, where he continued to play rugby and in which he participated in science and reflection clubs..
It was around this time that Rutherford's genius for experimentation began to manifest: his early investigations showed that iron could be magnetized by means of high frequencies, which in itself was a discovery. His excellent academic results allowed him to continue his studies and his research for a total of five years at that University. He graduated from Christchurch and shortly thereafter won New Zealand's only scholarship to study mathematics, supporting his final year by working as a teacher. He thus obtained the title of Master of Arts with the best marks in mathematics and physics.
In 1894 he obtained the title of Bachelor of Science, which allowed him to continue his studies in Great Britain, at the Cavendish Laboratories in Cambridge, under the direction of the discoverer of the electron, Joseph John Thomson from of 1895. He was the first overseas student to achieve this possibility. Before leaving New Zealand, he became engaged to Mary Newton, a young woman from Christchurch. At Cavendish Laboratories, he would replace Thomson years later.
Cambridge, 1895-1898
In the first place, he continued his research on hertzian waves and their reception at great distances. He gave an extraordinary presentation of his work to the Cambridge Physical Society, which was published in the Philosophical Transactions of the Royal Society, unusual for such a young researcher, which helped him to reach notoriety.
In December 1895, he began working with Thomson on the study of the effect of X-rays on a gas. They discovered that X-rays had the property of ionizing air, since they were able to show that it produced large numbers of charged particles, both positive and negative, and that these particles could recombine to give rise to neutral atoms. On his part, Rutherford invented a technique to measure the speed of ions and their rate of recombination. These jobs were what led him down the road to fame.
In 1898, after spending three years in Cambridge, when he was 27 years old, he was offered a professorship in physics at McGill University in Montreal, which he immediately accepted, since the professorship also represented for him the possibility of marrying his fiancée.
Montreal, 1898-1907: radioactivity
Henri Becquerel discovered around this time (1896) that uranium gave off an unknown radiation, "uranic radiation". Rutherford published an essential document in 1899, in which he studied the way these radiations could have to ionize the air, placing the uranium between two charged plates and measuring the current that passed. Thus, he studied the penetrating power of radiation, covering his uranium samples with metal sheets of different thicknesses. He noticed that the ionization started off rapidly as the thickness of the sheets increased, but that above a certain thickness it decreased more weakly. For this reason, he deduced that uranium emitted two different radiations, since they had different penetrating power. He called the less penetrating radiation alpha radiation, and the more penetrating (and necessarily producing less ionizing since it passed through the air) beta radiation.
In 1900, Rutherford married Mary Newton. From this marriage, his only daughter, Eileen, was born in 1901.
Around this time, Rutherford studies thorium and realizes, using the same device as for uranium, that opening a door in the laboratory significantly disturbs the experiment, as if air movements could alter the experiment. He will soon come to the conclusion that the thorium gives off an emanation, also radioactive, since by inhaling the air that surrounds the thorium, he realizes that this air easily transmits the current, even at a great distance from the thorium.
Note also that thorium fumes only remain radioactive for about ten minutes and are neutral particles. Its radioactivity is not altered by any chemical reaction, nor by changes in conditions (temperature, electric field). He also realizes that the radioactivity of these particles decreases exponentially, since the current passing between the electrodes also does, and thus discovers the period of radioactive elements in 1900. With the help of a Montreal chemist, Frederick Soddy, reached the conclusion in 1902 that the emanations of thorium were indeed radioactive atoms, but without being thorium, and that the radioactivity was accompanied by a disintegration of the elements.
This discovery caused a stir among chemists, who were very convinced of the principle of the indestructibility of matter. Much of the science of the time was based on this concept. Therefore, this discovery represents a true revolution. However, the quality of Rutherford's work left no room for doubt. It took Pierre Curie himself two years to accept this idea, despite the fact that he had already verified with Marie Curie that radioactivity caused a loss of mass in the samples. Pierre Curie was of the opinion that they lost weight without changing their nature.
Rutherford's research was recognized in 1903 by the Royal Society, which awarded him the Rumford Medal in 1904. He summarized the results of his research in a book entitled Radioactivity in 1904, in which he explained that radioactivity was not influenced by external conditions of pressure and temperature, nor by chemical reactions, but that it entailed an emission of heat greater than that of a chemical reaction. He also explained that new elements with different chemical characteristics were produced, while radioactive elements disappeared.
Together with Frederick Soddy, he calculated that the emission of thermal energy due to nuclear disintegration was between 20,000 and 100,000 times greater than that produced by a chemical reaction. He also launched the hypothesis that such energy could explain the energy released by the sun. They were of the opinion that if the earth maintains a constant temperature (as far as its core is concerned), it is undoubtedly due to the disintegration reactions that take place within it. This idea of a great potential energy stored in the atoms will find a confirmation principle a year later when Albert Einstein discovers the equivalence between mass and energy. After these works, Otto Hahn, the discoverer of nuclear fission along with Fritz Strassmann and Lise Meitner, will come to study with Rutherford at McGill for a few months.
Through numerous studies with radioactive elements, he observes that they emit two types of radiation. The first type of radiation, which he calls alpha rays, is highly energetic but has little range and is quickly absorbed by the medium. The second type of radiation is highly penetrating and far-reaching, which he calls beta rays. By using electric and magnetic fields he analyzes these rays and deduces their velocity, the sign of their charge, and the relationship between charge and mass. He also finds a third, highly energetic type of radiation, which he will call gamma rays.
Manchester, 1907-1919: the atomic nucleus
In 1907, he obtained a teaching position at the University of Manchester, where he worked alongside Hans Geiger. With this, he will invent a counter that allows the detection of alpha particles emitted by radioactive substances (prototype of the future Geiger counter), since by ionizing the gas found in the device, they produce a discharge that can be detected. This device allows them to estimate Avogadro's number in a very direct way: finding out the disintegration period of radium and measuring with their device the number of disintegrations per unit of time. In this way they deduced the number of radium atoms present in the sample.
In 1908, together with one of his students, Thomas Royds, he definitively proved what was supposed: that alpha particles are helium nuclei. In reality, what they prove is that once freed from their charge, alpha particles are helium atoms. To demonstrate this, he isolated the radioactive substance in a material thin enough for alpha particles to pass through effectively, but to do so by blocking any "emanation" of any kind. of radioactive elements, that is, any product of disintegration. He then collects the gas around the box containing the samples and analyzes its spectrum. He then finds a large quantity of helium: the nuclei that constitute the alpha particles have recovered available electrons.
That same year, he won the Nobel Prize in Chemistry for his work in 1908. He suffered, however, a little upset, since he basically considered himself a physicist. One of his most famous quotes is that "science is either Physics or philately", which undoubtedly placed physics above all other sciences.
In 1911 he made his greatest contribution to science, discovering the atomic nucleus. He had observed in Montreal by bombarding a thin sheet of mica with alpha particles, which produced a deflection of these particles. By revisiting these experiments more thoroughly, Geiger and Marsden, and using gold foil, found that some alpha particles deviated by more than 90 degrees. Rutherford then launched the hypothesis, which Geiger and Marsden confronted with the conclusions of their experiment, that in the center of the atom there must be a "nucleus"; that it contained almost all the mass and all the positive charge of the atom, and that in fact the electrons should determine the size of the atom. This planetary model had been suggested in 1904 by a Japanese, Hantarō Nagaoka, although it had gone unnoticed. He objected that in this case the electrons would have to radiate by revolving around the central nucleus and, consequently, fall. The results showed that this was undoubtedly the good model, since it allowed us to accurately predict the diffusion rate of alpha particles as a function of the diffusion angle and one order of magnitude for the dimensions of the atomic nucleus. The last theoretical objections (on the irradiation of the electron) vanished with the principles of quantum theory and Niels Bohr's adaptation of Rutherford's model to Max Planck's theory, which served to demonstrate the stability of Rutherford's atom.
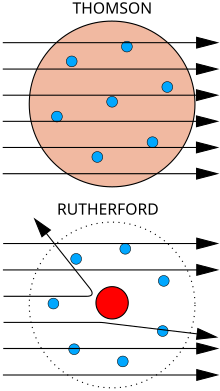
Down: Obervado results: a small proportion of particles were deflected, indicating a small concentrated load, that is to say the atomic nucleus. The diagram is not in scale; in reality the core is much smaller than the outer layer of electrons.
In 1914 World War I began, and Rutherford concentrated on acoustic methods of detecting submarines. After the war, already in 1919, he carries out the first artificial transmutation of him. After looking at the protons produced by hydrogen bombardment of alpha particles (by looking at the flicker they produce on screens covered in zinc sulfide), you realize you get a lot of those flickers if you do the same experiment with air and even more. with pure nitrogen. He deduces from this that the alpha particles, by striking the nitrogen atoms, have produced a proton, that is to say, that the nitrogen nucleus has changed its nature and has been transformed into oxygen, by absorbing the alpha particle. Rutherford had just produced the first artificial transmutation in history. Some are of the opinion that he was the first alchemist to achieve the goal of him.
Cambridge, 1919-1937: the golden age of Cavendish
That same year he succeeds J. J. Thomson in the Cavendish laboratory, becoming the director. It is the beginning of a golden age for the laboratory and also for Rutherford. From that time, his influence on research in the field of nuclear physics is enormous. For example, in a lecture that he gave to the Royal Society, he already alluded to the existence of the neutron and the isotopes of hydrogen and helium. And these will be discovered in the Cavendish laboratory, under his direction. James Chadwick, discoverer of the neutron (Nobel Prize in 1932 for this), Niels Bohr, who showed that Rutherford's planetary model was not unstable, and Robert Oppenheimer, who is considered the father of the atomic bomb, are among those who studied in the laboratory in Rutherford's time. Moseley, who was a student of Rutherford, showed, using the deflection of X-rays, that atoms had as many electrons as there were positive charges in the nucleus, and that it followed that his results "strongly confirmed Bohr's and Rutherford». John Cockcroft and Ernest Walton received the Nobel Prize in 1938 for an experiment demonstrating the disintegration of the atom using a particle accelerator, and Edward Appleton also received the Nobel Prize, in 1947, for demonstrating the existence of the ionosphere.
The large number of classes he gave in the Cavendish laboratory and the large number of contacts he had with his students gave an image of Rutherford as a person very attached to facts, even more than to theory, who for himself alone it was part of an "opinion." This adherence to experimental facts was the sign of great rigor and great honesty. When Enrico Fermi managed to disintegrate various elements with the help of neutrons, he wrote to congratulate him on having managed to "escape theoretical physics."
Fortunately, Rutherford did not stop at the facts, and his great imagination allowed him to glimpse, beyond, the most distant theoretical consequences, but he could not accept that things were needlessly complicated. He frequently made remarks to this effect to laboratory visitors who came to exhibit their work to students and researchers, whatever the fame of the visitor. His attachment to simplicity was almost proverbial. As he himself said: "I myself am a simple man."
His authority in the Cavendish lab was not based on fear he might inspire. By contrast, Rutherford had a jovial character. You knew he was making progress in his work when you could hear him humming in the lab. His students respected him greatly, not so much for his past work or the myth that surrounded him as for his attractive personality, his generosity, and his intellectual authority. His Russian disciple Peter Kapitza nicknamed him "the crocodile" and thus he was known among his colleagues. Not because it was fearsome or dangerous, but because for a Soviet so far from the African rivers, the concept of the crocodile represented tremendous power. Although no one would call him that up front, Rutherford knew it well and was secretly proud. Furthermore, the building built for Kapitza's studios had a large bas-relief of a crocodile.
This is also the time of honors for Rutherford: he was president of the Royal Society between 1925 and 1930, and chairman of the Academic Assistance Council, which in those politically turbulent times helped university students Germans fleeing their country. He was also awarded the Franklin Medal in 1924 and the Faraday Medal in 1936. He made his last trip to New Zealand, his native country, which he never forgot, in 1925 and was greeted as a hero. He rose to the peerage in 1931 and obtained the title of Baron Rutherford of Nelson, of Cambridge. But that same year his only daughter, Eileen, died nine days after giving birth to her fourth child.
Rutherford was a very robust man and entered the hospital in 1937 for a minor operation, after injuring himself trimming some trees on his property. Upon returning home, he seemed to be recovering without problems, but his condition suddenly worsened. He died on October 19 and was buried in Westminster Abbey, along with Isaac Newton and Kelvin.
Legacy
Rutherford is considered one of the greatest scientists in history.
Rutherford's research, and work done under his direction as director of the laboratory, established the nuclear structure of the atom and the essential nature of radioactive decay as a nuclear process. Patrick Blackett, a researcher working under Rutherford, demonstrated nuclear induced transmutation using natural alpha particles. Subsequently, Rutherford's team, using protons from an accelerator, demonstrated artificially induced nuclear reactions and transmutation. He is known as the father of nuclear physics. Rutherford died too soon to see Leó Szilárd's nuclear chain reaction idea materialize. However, a speech by Rutherford on his artificially induced transmutation into lithium, published in the London newspaper The Times on September 12, 1933, was cited by Szilárd as his inspiration to think about the possibility of a controlled nuclear chain reaction that produces energy. Szilard had this idea while walking around London that same day.
Rutherford's speech referred to work done in 1932 by his students John Cockcroft and Ernest Walton to "divide" lithium into alpha particles by bombarding a particle accelerator they had built with protons. Rutherford realized that the energy released by splitting lithium atoms was enormous, but he also realized that the energy required for the accelerator, and its essential inefficiency in splitting atoms in this way, made the project an impossibility. as a practical source of power (light element accelerator-induced fission is still too inefficient to be used in this way, even today). Rutherford's speech, in part, said
We could get in these processes much more energy than the proton provided, but in general we could not expect to get energy this way. It was a very poor and ineffective way of producing energy, and anyone who sought a source of energy in the transformation of the atoms was on the moon. But the subject was scientifically interesting because it allowed to know the atoms inside.
Top Posts
- Radio-activity (Cambridge University Press, 1904), (Reissued by Dover Phoenix Editions in 2005, ISBN 0-486-49585-X)
- Radioactive Transformations (1906), (Reissued by Juniper Grove in 2007, ISBN 1-60355-054-2)
- Radiations from Radioactive Substances (1919). Available at https://archive.org/details/radioactivesubst00ruthuoft
- The Electrical Structure of Matter (1926)
- The Artificial Transmutation of the Elements (1933)
- The Newer Alchemy (1937)
Eponymy
.
- Moon crater Rutherford carries this name in his memory.
- Martian crater Rutherford also commemorates his name.
- The asteroid (1249) Rutherfordia received this name in his honor.
- The Rutherfordius bears his name in memory of the scientist
Nobel Prize
| Predecessor: Eduard Buchner | Nobel Prize in Chemistry 1908 | Successor: Wilhelm Ostwald |
Rutherford received the 1908 Nobel Prize in Chemistry in recognition of "his investigations in the disintegration of the elements and in the chemistry of radioactive substances".
Among other distinctions, he was elected a member (1903) and president (1925-1930) of the Royal Society of London, and was granted the titles of sir in 1914 and baron Rutherford of Nelson in 1931.
Element 104 on the periodic table is named Rutherfordium in his honor.
On his death, his mortal remains were interred in Westminster Abbey.
Contenido relacionado
Ice
Weight
Electron cloud



