Enzyme
The enzymes They are organic molecules that act as catalysts for chemical reactions, that is, they accelerate the rate of reaction. They are commonly protein in nature, but also RNA (see ribozymes). Enzymes modify the rate of a reaction, without affecting its equilibrium, since an enzyme makes a chemical reaction proceed faster, as long as it is energetically possible (see Gibbs free energy). In these reactions, enzymes act on molecules called substrates, which are converted into different molecules called products. Almost all processes in cells need enzymes in order for them to occur at significant rates. Enzyme-mediated reactions are called enzymatic reactions.
Because enzymes are extremely selective with their substrates and their speed increases only with a few reactions, the set (set) of enzymes present in a cell determines the type of metabolism that cell has. In turn, this presence depends on the regulation of gene expression corresponding to the enzyme.
Like all catalysts, enzymes work by lowering the activation energy (ΔG‡) of a reaction, so the presence of the enzyme substantially speeds up the rate of reaction. Enzymes do not alter the energy balance of the reactions in which they are involved, nor do they modify, therefore, the equilibrium of the reaction, but they manage to speed up the process even on scales of millions of times. A reaction that occurs under the control of an enzyme, or a catalyst in general, reaches equilibrium much faster than the corresponding uncatalyzed reaction.
As ciliary occurs with other catalysts, enzymes are not consumed in the reactions they catalyze, nor do they alter their chemical balance. However, enzymes differ from other catalysts by being more specific. There is a great diversity of enzymes that catalyze around 4000 different biochemical reactions. Not all biochemical catalysts are proteins, since some RNA molecules are capable of catalyzing reactions (such as the 16S subunit of ribosomes in which peptidyl transferase activity resides).. It is also worth mentioning some synthetic molecules called artificial enzymes capable of catalyzing chemical reactions like classical enzymes.
The activity of enzymes can be affected by other molecules. Enzyme inhibitors are molecules that decrease or prevent enzyme activity, while activators are molecules that increase enzyme activity. Likewise, a large number of enzymes require cofactors for their activity. many drugs are inhibitory molecules. Likewise, the activity is affected by temperature, pH, the concentration of the enzyme itself and of the substrate, and other physicochemical factors.
Many enzymes are used commercially, for example, in the synthesis of antibiotics or household cleaning products. In addition, they are widely used in various industrial processes, such as the manufacture of food, the destination of jeans or the production of biofuels.
Etymology and history
Since the late 18th century and early XIX, the digestion of meat by stomach secretions was known, and the conversion of starch into sugar by plant extracts and saliva. However, the underlying mechanism had not been identified. The first enzyme was discovered by Anselme Payen and Jean-François Persoz in 1833.
In the 19th century, when the fermentation of sugar in alcohol with yeast was being studied, Louis Pasteur came to the conclusion that this fermentation was catalyzed by a vital force contained in the yeast cells, called ferments, and initially it was thought that they only worked with living organisms. He wrote that "the fermentation of alcohol is an act related to the life and organization of yeast cells, and not to the death and putrefaction of cells". On the contrary, other scientists of the time, such as Justus von Liebig, remained in the position that defended the purely chemical nature of the fermentation reaction.
In 1878 the physiologist Wilhelm Kühne (1837-1900) coined the word «enzyme», which comes from the Greek ενζυμον, «in yeast», to describe this process. The word enzyme was later used to refer to inert substances such as pepsin. On the other hand, the word "ferment" used to refer to the chemical activity produced by living organisms.
In 1897 Eduard Buchner began to study the ability of yeast extracts to ferment sugar despite the absence of living yeast cells. In a series of experiments at the Humboldt University of Berlin, he found that sugar was fermented even when there were no living elements in the yeast cell cultures. He named the enzyme that causes sucrose fermentation "zymase". In 1907 he received the Nobel Prize in Chemistry "for his biochemical research and for having discovered cell-free fermentation". Following Buchner's example, enzymes are usually named according to the reaction they produce. Normally, the suffix "-asa" it is added to the name of the substrate (eg, lactase is the enzyme that breaks down lactose) or to the type of reaction (eg, DNA polymerase forms DNA polymers).
Having shown that enzymes can function outside of a living cell, the next step was to determine their biochemical nature. In much of the early work it was noted that enzyme activity was associated with proteins, but some scientists (such as Nobel laureate Richard Willstätter) argued that proteins were simply the transport for the actual enzymes and that proteins per se were not capable of catalysis. However, in 1926, James B. Sumner demonstrated that the urease enzyme was a pure protein and crystallized it. Summer did the same with the enzyme catalase in 1937. The conclusion that pure proteins could be enzymes was definitively proven by John Howard Northrop and Wendell Meredith Stanley, who worked with various digestive enzymes such as pepsin (1930), trypsin, and chymotrypsin. These three scientists received the Nobel Prize in Chemistry in 1946.
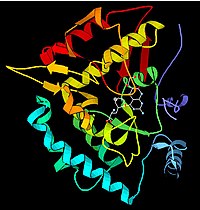
The discovery that enzymes could be crystallized allowed their structures to be resolved using crystallography and X-ray diffraction techniques. This was done first with lysozyme, an enzyme found in tears, saliva, and the eggs, capable of digesting the wall of some bacteria. The structure was solved by a group led by David Chilton Phillips and published in 1965. This high-resolution structure of lysozymes marked the beginning of the field of structural biology and the effort to understand how enzymes work in the molecular order..
Structures and mechanisms
Enzymes are generally globular proteins that can have very variable sizes, from 62 amino acids, as in the case of the monomer of 4-oxalocrotonate tautomerase, to the 2,500 present in fatty acid synthase.
The activities of enzymes are determined by their three-dimensional structure, which is in turn determined by the amino acid sequence. However, while structure determines function, predicting new enzyme activity based on the structure of the enzyme alone a protein is very difficult, and a problem not yet solved.
Almost all enzymes are much larger than the substrates they act on, and only a small part of the enzyme (about 3–4 amino acids) is directly involved in catalysis. The region containing these residues in charge to catalyze the reaction is called active site. Enzymes may also contain sites with the ability to bind cofactors, sometimes necessary in the catalytic process, or to bind small molecules, such as substrates or products (direct or indirect) of the catalyzed reaction. These unions of the enzyme with its own substrates or products can increase or decrease enzyme activity, thus giving rise to a regulation by positive or negative feedback, depending on the case.
Like other proteins, enzymes are made up of a linear chain of amino acids that fold during the translation process to give rise to a three-dimensional tertiary structure of the enzyme, capable of presenting activity. Each amino acid sequence is unique and therefore gives rise to a unique structure, with unique properties. Sometimes individual proteins can join other proteins to form complexes, in what is called quaternary protein structure.
Most enzymes, like the rest of the proteins, can be denatured if they are subjected to denaturing agents such as heat, extreme pHs or certain compounds such as SDS. These agents destroy the tertiary structure of proteins reversibly or irreversibly, depending on the enzyme and the condition. A consequence of denaturation is the loss or reduction of the function, of the enzymatic capacity.
Specificity
Enzymes are usually very specific both to the type of reaction they catalyze and to the substrate involved in the reaction. The shape, charge, and hydrophilic/hydrophobic characteristics of enzymes and substrates are responsible for this specificity. The specificity constant is a measure of the efficiency of an enzyme, since the rate of the reaction is directly related to the frequency with which enzyme and substrate molecules meet. Enzymes can also display a high degree of stereospecificity, regioselectivity, and chemoselectivity.
Some of these enzymes that show high specificity and precision in their activity are those involved in genome replication and expression. These enzymes have efficient error checking and correction systems, as in the case of DNA polymerase, which catalyzes a replication reaction in a first step, to later check if the product obtained is correct. This process, which takes place in two steps, results in an incredibly low average error rate, around 1 error per 100 million reactions in certain mammalian polymerases. Such checking mechanisms have also been observed in RNA polymerase, in tRNA aminoacyl synthetase and the selection activity of aminoacyl-tRNAs.
Those enzymes that produce secondary metabolites are called promiscuous, since they can act on a wide variety of substrates. Therefore, it has been suggested that this broad substrate specificity could be key in the evolution and design of new biosynthetic pathways.
"Key-lock" model
Enzymes are very specific, as Emil Fischer suggested in 1894. Based on his results, he deduced that both molecules, the enzyme and its substrate, possess geometric complementarity, that is, their structures fit exactly into one another, for This model has been called the "lock-key" model, referring to the enzyme as a kind of lock and the substrate as a key that fits perfectly into said lock. A key only works in its lock and not in other locks. However, while this model explains the specificity of enzymes, it fails to explain the stabilization of the transition state that enzymes manage to acquire.
The complementarity between the enzyme and the substrate results from the stereochemical adjustment of the substrate in the active site, for which both structures must be electrostatically complementary. In this model, a three-point anchoring is postulated, which returns selective enzymatic catalysis to three levels:
- Estereoméricabecause it discriminates between different stereoisomers of the substrate.
- Regiomerica, because it binds between specific regions of both the enzyme and the substrate.
- Enantiomericabecause the enzyme also discriminates against enantiomers.
Induced lace model
In 1958, Daniel Koshland suggested a modification to the lock-key model: enzymes are fairly flexible structures and thus the active site could change its structural conformation by interaction with the substrate. As a result, the chain The amino acid that makes up the active site is molded into precise positions, allowing the enzyme to carry out its catalytic function. In some cases, such as glycosidases, the substrate changes shape slightly to enter the active site. The active site continues this change until the substrate is fully bound, at which point the final shape and charge are determined.
Conformational changes are important in other processes besides catalysis, such as the maintenance of the quaternary structure, since the formation or disassembly of multimeric complexes depends on the contact between protein binding domains. If a conformation is the only one compatible for the quaternary structure, then the existence of mechanisms that regulate the stability of multienzyme complexes is possible.
Mode of action
Enzymes can act in various ways, as will be seen below, always leading to a decrease in the value of ΔG‡:
- Reduced activation energy by creating an environment in which the state of transition is stabilized (e.g., forcing the form of a substrate: the enzyme produces a change of substrate conformation which passes to a state of transition, so that it sees reduced the amount of energy needed to complete the transition).
- Reducing the energy of the state of transition, without affecting the shape of the substrate, by creating an environment with an optimal load distribution to generate such a state of transition.
- Providing an alternative route. For example, temporarily reacting with the substrate to form an intermediate enzyme/sustrate complex (ES), which would not be feasible in the absence of an enzyme.
- Reducing the variation of entropy necessary to achieve the state of transition (activation energy) of the reaction through the action of correctly orienting the substrates, thus favoring the reaction.
- Increase the speed of the enzyme by increasing temperature. The temperature increase facilitates the action of the enzyme and allows its reaction rate to increase even more. However, if the temperature rises too high, the structural conformation of the enzyme can be affected, thus reducing its reaction rate, and only recovering its optimal activity when the temperature is reduced. However, some enzymes are thermal and work better at low temperatures.
Note that this entropic effect involves destabilization of the ground state, and its contribution to catalysis is relatively small.
Stabilization of the transition state
Understanding the origin of the reduction in the value of ΔG‡ in an enzymatic reaction requires previously elucidating how enzymes can stabilize their transition state, rather than the transition state of the reaction. Apparently, the most effective way to achieve stabilization is the use of electrostatic forces, namely, having a relatively fixed polar environment that can be oriented towards the transition state charge distribution. Such environments do not exist or are generated in the absence of enzymes.
Function
The internal dynamics of enzymes is related to their catalytic mechanisms. Internal dynamics is defined as the movement of different parts of the enzyme structure, from individual amino acid residues, to groups of amino acids, or even an entire protein domain. These movements occur at different time scales ranging from femtoseconds to seconds. Almost any residue of the enzyme structure can contribute to the catalytic process through dynamic movements. Protein movements are vital in many enzymes. These movements may be more or less important depending on whether the conformational changes are produced by small and fast or large and slow vibrations, and this importance will depend on the type of reaction carried out by the enzyme. However, while these movements are important in the process of binding and releasing substrates and products, it is not yet clear whether these movements help speed up the chemical steps of enzymatic reactions. These new advances also have implications for understanding the allosteric effects and in the development of new drugs.
Allosteric modulation
Allosteric sites are areas of the enzyme with the ability to recognize and bind certain molecules in the cell. The unions to which they give rise are weak and non-covalent, and they generate a change in the structural conformation of the enzyme that affects the active site, thus affecting the reaction rate. Allosteric interactions can both inhibit and activate enzymes, and are a very common way to control enzymes in cells.
Cofactors and coenzymes
Cofactors
Some enzymes do not require any additional component to show full activity. However, other enzymes require the binding of non-protein molecules called cofactors in order to exert their activity. Cofactors can be inorganic compounds, such as metal ions and ferrosulfur complexes, or organic compounds, such as flavin or heme. Organic cofactors can be either prosthetic groups, which bind strongly to the enzyme, or coenzymes, which are released from the active site of the enzyme during the reaction. Coenzymes include compounds such as NADH, NADPH, and adenosine triphosphate. These molecules transfer functional groups between enzymes.
An example of an enzyme that contains a cofactor is carbonic anhydrase, in which zinc (cofactor) remains bound to the active site, as shown in the figure above (located at the beginning of section &# 34;Structures and Mechanisms"). These molecules are usually found attached to the active site and are involved in catalysis. For example, flavin and the heme group are often involved in redox reactions.
Enzymes that require a cofactor but do not have it attached are called "apoenzymes" or "apoproteins." An apoenzyme together with cofactor(s) is called a "holoenzyme" (which is the active form). Most cofactors do not bind covalently to their enzymes, but they do bind strongly. However, prosthetic groups may be covalently attached, as in the case of thiamine pyrophosphate in the enzyme pyruvate dehydrogenase. The term "holoenzyme" can also be applied to those enzymes that contain multiple subunits, as in the case of DNA polymerase, where the holoenzyme is the complex with all the subunits necessary to carry out the enzymatic activity.
Coenzymes
Coenzymes are small organic molecules that carry chemical groups from one enzyme to another. Some of these compounds, such as riboflavin, thiamine, and folic acid, are vitamins (which cannot be synthesized in sufficient amounts by the body human and must be incorporated into the diet). Exchanged chemical groups include the hydride ion (H-) carried by NAD or NADP+, the phosphate group carried by ATP, the acetyl group carried by coenzyme A, formyl, methenyl, or methyl groups carried by folic acid and the methyl group carried by S-Adenosyl methionine.
Because coenzymes undergo chemical modification as a consequence of enzymatic activity, it is useful to consider coenzymes as a special class of substrates, or second substrates, that are common to many different enzymes. For example, about 700 enzymes that use the coenzyme NADH are known.
Coenzymes are usually continually regenerating and their concentrations are usually kept at fixed levels inside the cell: for example, NADPH is regenerated via the pentose phosphate and S-Adenosyl methionine by means of methionine adenosyltransferase. This continuous regeneration means that even small amounts of coenzymes are used intensively. For example, the human body expends its own weight in ATP each day.
The high price of NADH and NADPH is an economic drawback in the use of coenzyme-dependent enzymes in industrial biotechnological processes. Therefore, the development of cheap biomimetic cofactors is of strategic importance.
Thermodynamics
As with all catalysts, enzymes do not alter the chemical equilibrium of the reaction. Generally, in the presence of an enzyme, the reaction proceeds in the same direction that it would in the absence of an enzyme, only faster. However, in the absence of enzyme, a spontaneous reaction could occur that would generate a different product because under those conditions, said different product is formed more quickly.
In addition, enzymes can couple two or more reactions, so a thermodynamically favorable reaction can be used to promote another thermodynamically unfavorable reaction. For example, ATP hydrolysis is often used to promote other chemical reactions.
Enzymes catalyze chemical reactions both in one direction and in the opposite direction. They never alter the balance, but only the speed at which it is reached. For example, carbonic anhydrase catalyzes its reaction in one direction or another depending on the concentration of the reactants, as can be seen below:
- CO2+H2O→.H2CO3{displaystyle mathrm {CO_{2}+H_{2}O{xrightarrow {.}H_{2}CO_{3}}} } (in tissues; high CO concentration2)
- H2CO3→.CO2+H2O{displaystyle mathrm {H_{2}CO_{3}{xrightarrow {.}CO_{2}{2}O} } (in lungs; low CO concentration2)
If the equilibrium is shifted too much in one direction of the reaction, that is, it becomes a very exergonic reaction, the reaction becomes effectively irreversible. Under these conditions, the enzyme will only catalyze the reaction in the direction allowed from a thermodynamic point of view.
Kinetics
Enzyme kinetics is the study of how enzymes bind to their substrates and transform them into products. Equilibrium data used in kinetic studies are obtained by enzymatic assays.
In 1902, Victor Henri proposed a quantitative theory of enzyme kinetics, but his experimental data were not very useful because the importance of the hydrogen ion concentration was not yet considered. After Peter Lauritz Sørensen defined the logarithmic scale of pH and introduced the concept of "buffer" (chemical buffer) in 1909, German chemist Leonor Michaelis and her Canadian postdoc Maud Leonora Menten repeated Henri's experiments confirming his equation, which it is now known as Henri-Michaelis-Menten kinetics (or simply Michaelis-Menten kinetics). His work was further developed by George Edward Briggs and J. B. S. Haldane, who derived the kinetic equations that are so widely used in science. present.
Henri's biggest contribution was the idea of dividing enzymatic reactions into two stages. In the first, the substrate reversibly binds to the enzyme, forming the enzyme-substrate complex (also called the Michaelis complex). In the second, the enzyme catalyzes the reaction and releases the product.
Enzymes can catalyze up to several million reactions per second. For example, the non-enzymatic decarboxylation of orotidine 5'-monophosphate (EC 2.4.2.10) has a half-life of 78 million years. However, when the enzyme orotidine 5'-phosphate decarboxylase is present in the medium, that same process takes as little as 25 milliseconds. Enzyme rates depend on solution conditions and substrate concentration. Those conditions that denature a protein, such as high temperatures, extreme pH or high salt concentrations, hinder or prevent enzyme activity, while high substrate concentrations tend to increase activity. To find the maximum rate of an enzymatic reaction, the substrate concentration is increased until a constant rate of product formation is obtained (see the saturation curve depicted in the figure on the right). Saturation occurs because as the concentration of substrate increases, the concentration of free enzyme decreases, which is converted to the substrate-bound (ES) form. At the maximum rate (Vmax) of the enzyme, all active sites of that enzyme have bound substrate, and the number of complexes is equal to the total amount of enzyme.. However, Vmax is only one of the kinetic constants of the enzyme. The amount of substrate needed to obtain a given reaction rate is also important. This parameter is given by the Michaelis-Menten constant (Km), which is the substrate concentration necessary for an enzyme to reach half its maximum speed.. Each enzyme has a characteristic Km value for a given substrate, which can tell us how close the binding between the substrate and the enzyme is. Another useful constant is the turnover number, kcat, which is the number of substrate molecules processed by each active site per second.
The efficiency of an enzyme can be expressed in terms of kcat/Km, where which is called the specificity constant, which incorporates the rate constant of all phases of the reaction. Since the specificity constant contemplates both affinity and catalytic capacity, it is a very useful parameter to compare different enzymes or the same enzyme with different substrates. The theoretical maximum value of the specificity constant is called the diffusion limit and has a value of 108-109 (M−1 s−1). At this point, each collision of the enzyme with its substrate leads to catalysis, so the rate of product formation is not limited by the reaction rate, but rather by the diffusion rate. Enzymes that possess this property are called catalytically perfect enzymes or kinetically perfect enzymes. Examples of this type of enzyme are triose phosphate isomerase, carbonic anhydrase, acetylcholinesterase, catalase, fumarase, beta-lactamase, and superoxide dismutase.
Michaelis-Menten kinetics depends on the law of mass action, which is derived from the assumptions of free diffusion and random collision. However, many biochemical or cellular processes deviate significantly from these conditions, due to phenomena such as macromolecular crowding, enzyme-substrate-product stage separation, or one- or two-dimensional molecular motions. situations a Michaelis-Menten fractal kinetics can be applied.
Some enzymes have kinetics faster than the rate of diffusion, which at first seems impossible. Various mechanisms have been proposed to try to explain this phenomenon. One of the models proposes that some proteins could have the ability to accelerate catalysis by sequestering the substrate and orienting it by dipolar electric fields. Another model proposes a quantum tunneling mechanism, where a proton or an electron can tunnel through activation barriers, although there is some controversy as to whether a proton can tunnel. Proton-mediated tunneling has been observed in tryptamine. This suggests that enzyme catalysis could be more accurately defined as a "barrier", rather than the traditional model, where the substrate requires the enzyme to reach a lower energy barrier.
Inhibition
Inhibitors are molecules that alter the kinetic parameters of an enzymatic reaction and therefore regulate enzyme activity. Broadly speaking, they can be classified as reversible and irreversible. The irreversible ones bind covalently to the enzyme without the possibility of reversing the modification, being useful in pharmacology. Some of the drugs that work in this way are eflornithine, used to treat African trypanosomiasis, penicillin and aspirin.
The reversible ones bind reversibly to the enzyme, and can be classified, in turn, according to the way in which they intervene in the reaction, as competitive, acompetitive and mixed. Usually, due to its widespread presence in a multitude of processes, it is also referred to as non-competitive inhibition, which in reality is nothing more than a variant of the already mentioned mixed inhibition. However, due to its characteristics it is usually presented as opposed to the competitive one, with which it is frequently compared.
- In the competitive inhibition, substrate (S) and inhibitor (I) cannot be joined to the same enzyme at the same time, as shown in the figure of the right. This usually occurs when the inhibitor has affinity for the active site of an enzyme in which the substrate is also joined; the substrate and the inhibitor compiten for access to the active site of the enzyme. For example, metotrexate is a competitive inhibitor of the enzyme dihydrofolate reductase, which catalyzes the reduction of dihydrofolate to tetrahydrofolate. The similarity between the structures of folic acid and metotrexate allows a competitive inhibition to be established. This type of inhibition can be overcome with sufficiently high substrate concentrations, i.e., leaving the inhibitor out of competition. In competitive inhibition the maximum speed of reaction does not vary, but higher substrate concentrations are needed to achieve a certain speed, thus increasing Km apparent.
- In the acompetitive inhibition inhibitor cannot join the free enzyme, but only the enzyme-sustrate complex (ES). Once the complex is formed with the inhibitor (EIS) the enzyme is inactive. This type of inhibition is rare, but may occur in multimetic enzymes.
- La non-competitive inhibition is a form of mixed inhibition where the binding of the inhibitor with the enzyme reduces its activity but does not affect the union with the substrate. As a result, the degree of inhibition depends only on the concentration of inhibitor, regardless of the substrate concentration, thus varying the value of Vmax apparent. However, as the substrate can still join the enzyme, the value of Km It doesn't vary.
- In the Mixed inhibition, the inhibitor can join the enzyme at the same time as the substrate. However, the union of the inhibitor affects the union of the substrate, and vice versa. This type of inhibition can be reduced, but not overcome by increasing substrate concentrations. Although it is possible that mixed-type inhibitors join the active site, this type of inhibition usually results from an allergy effect where the inhibitor binds to another site that is not the active site of the enzyme. The binding of the inhibitor with the alostic site changes the conformation (i.e. the tertiary structure) of the enzyme so that the affinity of the substrate by the active site is reduced.
In many organisms, inhibitors can act as part of a feedback mechanism. If an enzyme produces too much of a substance in the body, this same substance could act as an inhibitor of the enzyme at the beginning of the pathway that produces it, thus stopping its production when there is enough of the substance in question. This would be a form of negative feedback. Enzymes that are subject to this type of regulation are usually multimeric and have allosteric sites where regulatory substances bind. The graphs representing the rate of the reaction against the substrate concentration of these enzymes are not hyperbolic, but rather sigmoidal (S-shaped).
- Uses of inhibitors
Because inhibitors modulate enzyme function, they are often used as drugs. A typical example of an inhibitor that is used as a drug is aspirin, which inhibits the COX-1 and COX-2 enzymes involved in the synthesis of an inflammatory intermediate, prostaglandins, thus suppressing the side effects, pain and inflammation. However, other enzyme inhibitors act like poisons. For example, cyanide is an irreversible inhibitor that binds to iron and copper atoms in the active site of animal cell cytochrome c oxidase (plants are resistant to cyanide), thus blocking cellular respiration.
Biological function
Enzymes have a wide variety of functions in living organisms. They are essential in signal transduction and regulation processes, normally through kinases and phosphatases. They are also capable of producing movement, as is the case of myosin by hydrolyzing ATP to generate muscle contraction or the movement of vesicles by medium of the cytoskeleton. Another type of ATPases in the cell membrane are ion pumps involved in active transport processes. In addition, enzymes are also involved in much more exotic functions, such as light production by luciferase in fireflies. Viruses may also contain enzymes involved in cell infection, such as the HIV virus integrase and reverse transcriptase, or viral delivery, such as the neuraminidase of the influenza virus.
An important function of enzymes is that they perform in the digestive system of animals. Enzymes such as amylases and proteases are capable of breaking down large molecules (starch or protein, respectively) into smaller ones, so that they can be absorbed in the intestine. Starch molecules, for example, which are too large to be absorbed, are broken down by various enzymes to smaller molecules such as maltose, and finally to glucose, which can be absorbed through the cells of the intestine. Different digestive enzymes are capable of breaking down different types of food. Ruminants that have a herbivorous diet have in their intestines a series of microorganisms that produce another enzyme, cellulase, capable of degrading the cellulose present in the cell wall of plants.
Several enzymes can act together in a specific order, thus creating a metabolic pathway. In a metabolic pathway, one enzyme takes the product of another enzyme as its substrate. After the catalytic reaction, the product is transferred to the next enzyme and so on. Sometimes, there is more than one enzyme capable of catalyzing the same reaction in parallel, which makes it possible to establish a more sophisticated regulation: for example, in the case in which one enzyme presents a constitutive activity but with a low activity constant and a second enzyme whose activity is inducible, but has a higher activity constant.
Enzymes determine the steps that these metabolic pathways follow. Without enzymes, metabolism would not occur through the same steps, nor would it be fast enough to meet the needs of the cell. In fact, a metabolic pathway like glycolysis could not exist without enzymes. Glucose, for example, can react directly with ATP so that it becomes phosphorylated on one or more carbons. In the absence of enzymes, this reaction would proceed so slowly as to be negligible. However, if the enzyme hexokinase that phosphorylates carbon 6 of glucose is added and the concentration of the mixture is measured in a short space of time, only glucose-6-phosphate can be found at significant levels. Therefore, the networks of metabolic pathways within the cell depend on the set of functional enzymes that they present.
Activity control
Enzyme activity can be controlled in the cell mainly in these five ways:
- Production of the enzyme (at the transcription or translation level): the synthesis of an enzyme may be favored or disadvantaged in response to certain stimuli received by the cell. This form of gene regulation is called enzymatic induction and inhibition. For example, bacteria could acquire resistance to antibiotics such as penicillin thanks to the induction of enzymes called beta-lactamasas, which hydrolyze the beta-lactamic ring of the penicillin molecule. Another example is the enzymes present in the liver called P450 oxidase cytochrome, which are of vital importance in the metabolism of drugs and drugs. Induction or inhibition of these enzymes may result in the occurrence of pharmacological interactions.
- Shareimentalization of the enzyme: enzymes can be located in different cell compartments, so that different metabolic paths can take place independently. For example, fatty acids are synthesized by a set of enzymes located in the cytosol, in the endoplasmic reticule and in the Golgi apparatus, and later, such fatty acids are used by another set of different enzymes as an energy source in the mitochondria, through β-oxidation.
- Enzymatic inhibitors and activators: enzymes can be activated or inhibited by certain molecules. For example, the final product of a metabolic route usually acts as an inhibitor of any of the enzymes involved in the first reactions of the route, thus establishing a negative feedback that regulates the amount of final product obtained by that route. This negative feedback mechanism effectively adjusts the synthesis speed of intermediate metabolites with the demand of the cell, and allows the distribution of materials and energy economically to avoid excess or shortage of final products. This enzyme control allows to maintain a relatively stable environment within living organisms.
- Post-rational modification of enzymes: enzymes can undergo various post-reduction modifications such as phosphorylation, miristoilation and glycosylation. For example, in the insulin response, phosphorylation of a multitude of enzymes, such as glucogen synthesis, is produced, which helps in controlling the synthesis or degradation of glucogen and allows the cell to respond to changes in blood sugar levels. Another example of post-rational modification is the degradation of the polypeptide chain. Chemotripsin, a digestive protein, is synthesized in an inactive form, chemotripyngen, in the pancreas and transported in this state to the stomach, where it will be activated. This prevents the enzyme from digesting the pancreas and other tissues through which it passes before reaching the stomach. This type of inactive precursor of an enzyme is called zimgen.
- Environmental-dependent activation: some enzymes can be activated when they move from one environment to another with different conditions, such as the passage of the cytoplasm-reducing environment to the oxidative environment of the periplasm, the passage of an environment with elevated pH to another with low pH, etc. For example, hemaglutinin of the flu virus is activated by a conformational change that occurs when the pH of the medium is sufficiently acidic, which occurs when the virus enters the inside of the cell through a lysosome.
Disease Implications
Because tight control of enzyme activity is necessary for homeostasis, any malfunction (mutation, increased or decreased expression, or deletion) of a single critical enzyme can lead to the development of a genetic disease. The importance of enzymes is evidenced by the fact that a lethal disease can be caused by the malfunction of only one type of enzyme out of thousands of types that exist in our body.
An example of this is the most common type of phenylketonuria. In this genetic disease, a single amino acid mutation occurs in phenylalanine hydroxylase, an enzyme that catalyzes the first reaction in the degradation pathway of phenylalanine and related compounds. As this enzyme is inactive, a series of products accumulate that end up giving rise to the appearance of mental retardation if treatment is not received.
Another example is when a mutation occurs in germline genes that encode enzymes involved in DNA repair. In this case, as the DNA of the cells is not adequately repaired, mutations accumulate that often lead to the development of various types of hereditary cancer, such as xeroderma pigmentosa.
Classification and nomenclature of enzymes
Enzymes receive common names that allude to the type of reaction on the substrate. The name of an enzyme is often derived from the substrate or chemical reaction it catalyzes, with the word ending in -ase. For example, lactase (EC 3.2.1.108) acts on lactose; alcohol dehydrogenase (EC 1.1.1.1) oxidizes alcohol ("dehydrogenates" it); DNA polymerase (EC 2.7.7.7) also comes from the reaction that it catalyzes, which consists of polymerizing DNA.
The nomenclature committee of the International Union of Biochemistry and Molecular Biology (IUBMB) recommends a set of rules for the classification of enzymes, consisting of the acronym EC at the beginning (for the acronym of Enzyme Commission), followed by four digits separated by dots. The first figure is a natural number that goes from 1 to 7 and indicates the type of reaction that it catalyzes. All enzymes receive a numerical code that identifies the substrates, the type of reaction they carry out, and some other relevant additional information. For example, the enzyme that transfers electrons between ferredoxin (a protein) and NADPH (a coenzyme) is coded EC 1.18.1.2 and is known as ferredoxin:NADP+ oxide reductase, among others. similar names. The seven classes of enzymes that are currently recognized are listed below:
- EC 1 Oxidorreductas: catalyse oxydorreduction reactions or redox reactions. These are the transfer of reducing equivalents between a donor and an acceptor. The precise transfer of a coenzyme like NAD+, NADP+or FAD, as an intermediary in the transfer of electrons. After catalytic action, these coenzymes are modified to their degree of oxidation, so they must be recycled before a new catalytic reaction is made. Examples: dehydrogenase, peroxidase.
- EC 2 Transfers: transfer functional groups (obtained from the rupture of certain molecules) to other receptor substances. They usually act in the interconversion processes of monosaccharides, amino acids, etc. Examples: transamines, kinases.
- EC 3 Hidrolasas: they catalyze hydrolysis reactions with the consequent obtaining monomers from polymers. They act in digestion of food, prior to other phases of its degradation. The word hydrolysis derived from hydro → 'water' and lisis → 'dissolution'. Examples: glycosidase, lipase, stears.
- EC 4 Liases: catalyse reactions in which H groups are eliminated or added2O, CO2 and NH3 to form a double link or add to a double link, or other reactions that involve an electron rearrangement. Examples: descarboxyilasas, phenylalanine liasa (EC 4.3.1.24).
- EC 5 Isomerasas: they act on certain molecules by obtaining or changing their functional isomers or position, that is, they catalyze the racemization and position changes of a group in a given molecule by obtaining isomeric forms. They usually act in interconversion processes. Example: epimerase (mutasa).
- EC 6 Leagues: catalyze the degradation or synthesis of the links called "forces" by linking to high-energy molecules such as ATP. Examples: syntheses, carboxylasas.
- EC 7 Translocations: They are integral membrane proteins that transfer a substrate (ion or molecule) from side 1 to side 2 of a membrane, and are subdivided into 4 subclasses, according to the force that drives the transfer reaction. Example: cytochrome-c oxidase (EC 7.1.1.9), or H exporter type P carrier+ (EC 7.1.2.1).
Industrial applications
Enzymes are used in the chemical industry, and in other types of industry, where the use of very specialized catalysts is required. However, enzymes are limited both by the number of reactions they can carry out and by their lack of stability in organic solvents and high temperatures. For this reason, protein engineering has become a very active area of research where enzymes with new properties are attempted, either through rational design or in vitro evolution. These efforts have begun to have some successes, obtaining some enzymes that catalyze reactions that do not exist in nature.
Below is a table with various industrial applications of enzymes:
| Implementation | Enzymes used | Uses |
| Food processing | Mushrooms and plants. | Production of sugars from starch, such as in the production of corn syrup. In baking oven, catalyze the breakage of starch of the flour in sugar. The fermentation of sugar carried out by yeasts produces the carbon dioxide that makes the dough "up". |
| Proteas | Cookies makers use them to reduce the amount of proteins in the flour. | |
| Baby food | Tripsina | To pre-digy baby-oriented food. |
| brewing beer | The enzymes of the barley are released during the grinding phase in brewing the beer. | Enzymes released degrade starch and protein to generate simple, amino acid and peptide sugars that are used by yeasts in the fermentation process. |
| Barley enzymes produced at industrial level | Widely used in brewing to replace the natural enzymes of barley. | |
| Amilasa, glucanasa and proteases | They digest polysaccharides and proteins in the malt. | |
| Betaglucanas and arabinoxilanasas | They improve the filtration of the must and beer. | |
| Amyloglucosidase and pululanasas | Production of low-calorie beer and adjustment of fermentation capacity. | |
| Proteas | They remove the turbidity produced during beer storage. | |
| Acetolactatodecarboxilasa (ALDC) | It increases the efficiency of fermentation by reducing diacetyl formation. | |
| Fruit juices | Cell phones, pectins | Cleared of fruit juices. |
| Dairy industry | Renine, derived from the stomach of young ruminant animals (such as calves and sheep). | Cheese production, used to hydrolyze proteins. |
| Enzymes produced by bacteria | Currently, more and more used in the dairy industry. | |
| Lipasas | It is introduced during the production process of the Roquefort cheese to favor ripening. | |
| Lactane | Lactose breaking in glucose and galactose. | |
| Digestion of meat | Papaine | Softening of meat used to cook. |
| Starch industry | Amilasas, amiloglucosides and glucoamilasas | Conversion of starch into glucose and various sugars invested. |
| Glucosa isomerasa | Conversion of fructose glucose during the production of corn syrup from substances rich in starch. These syrups enhance sweetener properties and reduce calories better than sucrose and maintain the same level of sweetness. | |
| Paper industry | Amilasas, xilanasas, cellulasas y ligninasas | Degradation of starch to reduce its viscosity, adding apprehension. Xilanase reduce the necessary bleaching for discoloration; the cellulose smooth the fibers, favor the drainage of water and promote the elimination of inks; the lipases reduce the darkness and the liignins remove the lignine to soften the paper. |
| Biofuel Industry | Cell phones | Used to degrade cellulose in sugars that can be fermented. |
| Lignins | Used to remove lignin residues. | |
| Biological detergents | Mostly proteases, produced extracellularly by bacteria. | Used to help eliminate protective clothing dyes in pre-washing conditions and in direct applications of liquid detergent. |
| Amilasas | Washers detergents to remove starch resistant waste. | |
| Lipasas | Used to facilitate the elimination of fatty and oily dyes. | |
| Cell phones | Used in biological softeners. | |
| Contact lens cleaners | Proteas | To remove protein remains from contact lenses and thus prevent infections. |
| Oil industry | Catalasa | To generate oxygen from peroxide, and thus turn the latex into a sparkling smell. |
| Photographic industry | Proteasa (office) | Dissolve the gelatin of the photographic films used, thus allowing the recovery of its content in silver. |
| Molecular biology | Restriction enzymes, ligase DNA and polymerase | Used to manipulate DNA through genetic engineering. Of great importance in pharmacology, agriculture, medicine and criminalism. Essentials for restrictive digestion and for polymerase chain reaction. |
Further reading
Etymology and History
Structure and mechanisms of enzymes
Thermodynamics
| Cintics and inhibition
Function and control of enzymes in cells
Conventions to assign names to enzymes
Industrial applications
|
Contenido relacionado
Nucleoid
Rosaceae
Peponid