Emulsion
An emulsion is a heterogeneous mixture of two immiscible liquids. One liquid (the dispersed phase) is dispersed in another (the continuous phase or dispersant phase). Many emulsions are oil/water, with dietary fats being one of the most common types of oils found in daily life. Examples of emulsions include butter and margarine, milk and cream, espresso, mayonnaise, the photosensitive side of photographic film, magma, and cutting oil used in metalworking. In the case of butter and margarine, the fat surrounds the water droplets (in a water-in-oil emulsion); in milk and cream the water surrounds the fat droplets (in an oil-in-water emulsion). In certain types of magma, globules of liquid ferronickel may be dispersed within a continuous phase of liquid silicate. The process in which emulsions are prepared is called emulsification.
Emulsions are part of a more generic class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsions tend to imply that both the dispersed and continuous phases are liquid.
An emulsion becomes either a water-in-oil emulsion or an oil-in-water emulsion depending on the volume fraction of both phases and the type of emulsifier. Bancroft's rule generally applies: emulsifiers and emulsifying particles tend to promote phase dispersion in which they do not dissolve very well; for example, proteins dissolve better in water than in oil so they tend to form oil-in-water emulsions (hence they encourage the dispersion of oil droplets through a continuous phase of water).
The basic color of the emulsions is white. If the emulsion is diluted, the Tyndall effect spreads the light and distorts the color to blue; if it is concentrated, the color is distorted towards yellow. This phenomenon can be easily seen by comparing skim milk (fat free or low fat) with cream (with high concentrations of milk fat). Microemulsions and nanoemulsions tend to be clear due to the small size of the dispersed phase.
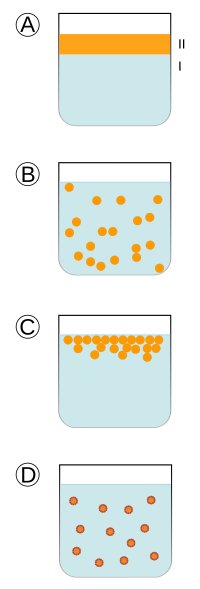
Appearance and properties
Definition IUPAC "Fluid system in which droplets of fluid are dispersed in a liquid. " Note 1: The definition is based on the definition of ref. Note 2: The drops can bemorphous, liquid-crystalline, or a mixture of both. Note 3: The diameters of the droplets that constitute the scattered phase They usually measure between 10 nm and 100 μm; that is, droplets may exceed the usual sizes of the colloidal particles. Note 4: An emulsion is called oil/water emulsion if the scattered phase is an organic material and the Continuous phase is water or an aqueous solution and is called water/oil if the scattered phase is water or an aqueous solution and the continuous phase is an organic liquid (a "oil"). Note 5: A water/oil emulsion is sometimes called an inverse emulsion. The term "inverse emulsion" is misleading, as it incorrectly suggests that emulsion has properties that are opposed to those of an emulsion. It is therefore not recommended for use. |
Emulsions contain both a dispersed and a continuous phase, and the boundary between the phases is called the "interface". Emulsions tend to appear cloudy because interfaces of many phases scatter light at as it passes through the emulsion. Emulsions appear white when all light is scattered equally. If the emulsion is dilute enough, high frequency (low wavelength) light will scatter more and the emulsion will appear more blue; this is called the "Tyndall effect". If the emulsion is sufficiently concentrated, the color will be distorted towards comparatively longer wavelengths and appear more yellow. This phenomenon is readily observable when skimmed milk, which contains little fat, is compared with cream, which contains a much higher concentration of milk fat. An example would be a mixture of water and oil.
Two special classes of emulsions, microemulsions and nanoemulsions, with droplet sizes below 100 nm, appear translucent. This property is due to the fact that light waves are scattered by the droplets only if their size exceeds approximately a quarter of the wavelength of the incident light. Since the visible spectrum of light is comprised of wavelengths between 390 and 750 nanometers (nm), if the droplet size in the emulsion is below about 100 nm, light can penetrate through the emulsion without disperse. Due to their similarity in appearance, translucent nanoemulsions and microemulsions are often confused. Unlike translucent nanoemulsions, which require specialized equipment to produce, microemulsions are spontaneously formed by "solubilizers" oil molecules with a mixture of co-surfactants, surfactants and co-solvents. The required concentration of surfactant in a microemulsion is, however, several times higher than in a translucent nanoemulsion, and significantly exceeds the concentration of the dispersed phase. Due to many undesirable side effects caused by surfactants, their presence is disadvantageous or prohibitive in many applications. Additionally, the stability of a microemulsion is often easily compromised by dilution, heating, or changing pH levels.
Ordinary emulsions are inherently unstable and therefore do not tend to form spontaneously. Energy input, through agitation, agitation, homogenization, or exposure to power ultrasound, is necessary to form an emulsion. Over time, emulsions tend to return to the steady state of the phases that make up the emulsion. An example of this is seen in the separation of the oil and vinegar components of vinaigrette, an unstable emulsion that will separate rapidly unless stirred almost continuously. There are important exceptions to this rule: microemulsions are thermodynamically stable, while translucent nanoemulsions are kinetically stable.
The fact that an oil and water emulsion becomes a "water in oil" or in an "oil in water" emulsion; depends on the volume fraction of both phases and the type of emulsifier (surfactant).
Instability
Emulsion stability refers to the ability of an emulsion to resist changes in its properties over time. There are four types of instability in emulsions: flocculation, coalescence, creaming/sedimentation, and Ostwald ripening. Flocculation occurs when there is a force of attraction between the droplets, so they form flocs, like bunches of grapes. This process may be desirable, if controlled to its extent, to adjust the physical properties of emulsions, such as their flow behavior. Coalescence occurs when droplets collide with each other and combine to form a larger droplet, thus that the average droplet size increases with time. Emulsions can also undergo creaming, where droplets rise to the top of the emulsion under the influence of buoyancy or under the influence of the centripetal force induced when a centrifuge is used. Creaming is a common phenomenon in dairy and non-dairy beverages (i.e. milk, almond milk, soy milk) and generally does not change droplet size. Sedimentation is the opposite phenomenon of creaming and is typically seen in water-in-oil emulsions. Sedimentation occurs when the dispersed phase is denser than the continuous phase and gravitational forces push the denser globules to the bottom of the emulsion. Similar to churning, sedimentation follows Stokes' law.
A "surfactant" An appropriate solution can increase the kinetic stability of an emulsion so that the size of the droplets does not change significantly with time. The stability of an emulsion, such as a suspension, can be studied in terms of the zeta potential, which indicates the repulsion between droplets or particles. If the size and dispersion of the droplets does not change with time, it is said to be stable. For example, oil-in-water emulsions containing mono- and diglycerides and milk protein as surfactant showed a droplet size of oil stable for 28 days of storage at 25 °C.
Monitoring physical stability
The stability of emulsions can be characterized using techniques such as light scattering, measurement of focused beam reflectance, centrifugation, and rheology. Each method has advantages and disadvantages.
Obtaining emulsions
An emulsion can be obtained from the following manipulations:
1. Physics: temperature increase (optional because there are also cold emulsifiers).
2. Chemical: through the action of emulsifiers.
3. Mechanics: through agitation.
Emulsifier
An emulsifier (also called an emulsifier) is a substance that stabilizes an emulsion, often a surfactant. Examples of emulsifying foods are egg yolk (where the main emulsifying chemical is lecithin), honey, and mustard, where a variety of chemicals in the mucilage around the seed pod act as emulsifiers; proteins and low molecular weight emulsifiers are the most common. In some cases, the particles can stabilize emulsions through a mechanism called Pickering stabilization.
Examples of food emulsifiers are:
- Egg yolk: where the main emulsifying and thickening agent is lecithin. Actually, lecithos It's the Greek word for egg yolk.
- Mustard [20] - where a variety of chemicals in the mucilage surrounding the seed shell act as emulsifiers
- Soy lecithin is another emulsifying and thickening.
- Pickering Stabilization: uses particles in certain circumstances.
- Sodium phosphates: it is not a direct emulsifier, [21] but modifies the behavior of other molecules, for example, casein.
- Mono and diglicérides: a common emulsifier found in many food products (coffee creams, ice creams, untar pastas, breads, cakes).
- Estearoil: sodium lactilate
- DATEM (monkey and digillide specimens of tartric diacetyl acid): an emulsifier that is used mainly in pastry.
- Simple cellulose: a particulate emulsifier derived from plant material that uses only water.
- Proteins: those with hydrophilic and hydrophobic regions, for example, sodium cassation as in the foundible cheese product.
Both mayonnaise and hollandaise sauce are oil-in-water emulsions that are stabilized with lecithin from egg yolks. Detergents are another class of surfactant, and can chemically interact with both oil and water, thus stabilizing the interface between suspended oil or water droplets. This principle is exploited in soap by removing grease for the purpose of cleaning. A wide variety of emulsifiers are used in the pharmacy to prepare emulsions such as creams and lotions. Common examples include emulsifying wax, cetearyl alcohol, polysorbate 20, and cetearet 20.
Sometimes the internal phase itself can act as an emulsifier, and the result is a nanoemulsion – the internal state is dispersed into nanometer-sized droplets within the external phase. A known example of this phenomenon occurs when water is poured into a high-alcohol beverage based on anise, such as ouzo, pastis or raki. The anisolic components, which are soluble in ethanol, form nanometer-sized droplets and emulsify within water. The color of this diluted drink is opaque and milky.
In the healthcare field
In medicine, microscopic emulsions are used to deliver vaccines and kill microbes. Typically, the emulsions used in these techniques are soybean oil nanoemulsions, with particles 400 to 600 nanometers in diameter. The process is not chemical, like other types of antipathogenic treatments, but rather physical. The droplets are small and have a high surface tension, therefore the binding force with other lipids is greater. The oil is emulsified with detergents to stabilize the emulsion (the droplets cannot bind to each other), so they encounter another class of lipids with a bacterial membrane or some virus shell, forcing the lipids to bind to each other. the others. On a mass scale, it effectively disintegrates the membrane and kills the pathogen.
It should be noted that the soybean oil emulsion does not harm normal human cells or the cells of most higher organisms. The exceptions are sperm and blood cells, which are vulnerable to nanoemulsions due to their membrane structures. For this reason, nanoemulsions of this type are not used in IVs.
The most effective application of this type of nanoemulsion is for the disinfection of surfaces. Some types of nanoemulsions have been shown to be effective in destroying HIV-1 and several tuberculosis pathogens, for example, on non-porous surfaces.
Phytosanitary products
Chemically in an emulsion we speak of a "dispersed phase" and another "dispersant phase", for example, at home we can disperse cooking oil in tap water and we obtain an emulsion, the dispersed phase would be the oil and the water would be the dispersant. We can also find emulsions in which water acts as a dispersed phase. Emulsions are unstable and the two phases, dispersant and dispersant, tend to separate. To avoid this in the formulation of this type of phytosanitary products, the use of one or several emulsifiers is frequent, which give stability to the mixture, avoiding the separation of the phases. The action of these emulsifiers can be altered by the type of water used, especially if we use hard water. EW stands for oil-in-water emulsion and EC stands for emulsifiable concentrate.
Contenido relacionado
Lactose
Ethanol
Saccharose