Electronic relocation
The electron delocalization, in physics and chemistry, is the phenomenon that occurs when one or several electrons that are not associated with a single atom or covalent bond can be distributed or move between several centers (for example, atoms in a molecule, ions, or metals). The term offshoring is general and can have slightly different meanings in different fields. In organic chemistry, the term delocalization is associated with resonance in conjugated and aromatic systems. In solid state physics, the term refers to the free electrons that facilitate electrical conductivity.
According to quantum mechanics, all the electrons in a system are equivalent and indistinguishable, and have no path, so it is not strictly correct to say that there are N pairs of electrons in a molecule localized and m roaming or delocalized electrons. However, the concept of electronic relocation is very useful to describe and rationalize certain types of systems.
Resonance
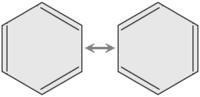
In the aromatic ring of benzene, the delocalization of six π electrons on the ring is usually indicated graphically as a circle. The fact that the six carbon-carbon bonds are equidistant is an indication that the electrons are delocalized. If the structure had double bonds independent of the single bonds, the bond lengths would be alternately longer or shorter. In valence bond theory, delocalization in benzene is represented by resonant structures.
Electric driving
Delocalized electrons also exist in the structure of solid metals. Metallic structures consist of cations aligned in a "sea" delocalized electrons. This means that electrons move freely through the entire structure, giving rise to properties such as conductivity.
In diamond, the four electrons in the valence shell of each carbon atom are located between the atoms in a covalent bond. The movement of the electrons is restricted and therefore the diamond does not conduct electricity. However, in graphite, each carbon atom uses only three of its four valence electrons to covalently bond with three other carbons that lie on the plane. Each carbon atom contributes one electron to give rise to a delocalization system that is also part of the chemical bond. The delocalized electrons can move through the entire plane, so graphite does conduct electricity through the plane formed by the carbon atoms, but it does not conduct electricity perpendicular to the plane.
Contenido relacionado
Ammonium
Donald glaser
Endemism