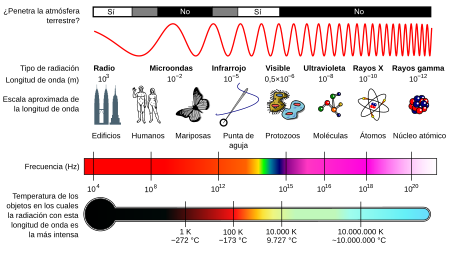
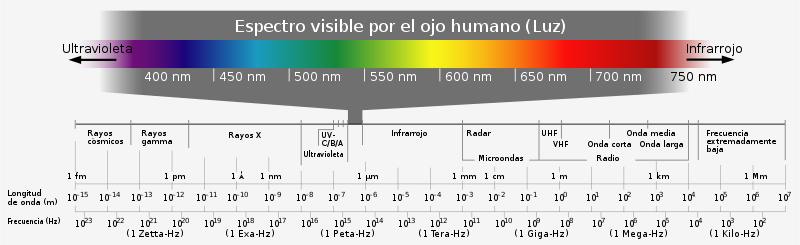
Electromagnetic spectrum
In physics, the electromagnetic spectrum refers to the energy distribution of all electromagnetic waves. Referring to an object, the electromagnetic radiation that a substance emits (emission spectrum) or absorbs (absorption spectrum) is called electromagnetic spectrum or simply spectrum. Such radiation serves to identify the substance in a manner analogous to a fingerprint. The spectra can be observed using spectroscopes that, in addition to allowing the spectrum to be seen, allow measurements to be made on it, such as the wavelength, frequency and intensity of the radiation.
The electromagnetic spectrum extends from radiation of shorter wavelengths, such as gamma rays and X-rays, through ultraviolet radiation, visible light and infrared radiation, to electromagnetic waves of longer wavelengths, as are radio waves. Although the limit for the smallest possible wavelength would not be the Planck length (because the characteristic time of each mode of interaction is about 1020 times greater than the Planck instant and, in the present cosmological stage, none of them could oscillate with the frequency necessary to reach that wavelength), it is believed that the maximum limit would be the size of the Universe (see Physical Cosmology) although formally the electromagnetic spectrum is infinite and continuous.
Energy range of the spectrum
The electromagnetic spectrum covers many different wavelengths. There are frequencies of 30 Hz and less that are relevant in the study of certain nebulae. On the other hand, frequencies close to 2.9×1027 Hz are known, which have been detected from astrophysical sources.
Electromagnetic energy at a particular wavelength λ (in a vacuum) has an associated frequency f and a photon energy E. Therefore, the electromagnetic spectrum can equally be expressed in any of these terms. They are related in the following equations:
c=fλ λ {displaystyle c=flambda ,!}or what is the same λ λ =cf{displaystyle lambda ={frac {c}{f}},!}
E=hf{displaystyle E=hf,!}or what is the same E=hcλ λ {displaystyle E={frac {hc}{lambda }{,}
Where c=299.792.458m/s{displaystyle c=299.792.458 mathrm {m/s} ,} (light speed) and h{displaystyle h,!} It's Planck's constant, (h≈ ≈ 6,626069⋅ ⋅ 10− − 34J⋅ ⋅ s≈ ≈ 4,13567μ μ eV/GHz){displaystyle (happrox 6,626069cdot 10^{-34} {mbox{J}}}cdot {mbox{s}}approx 4,13567 mathrm {mu } {mbox{eV}}}{{{mbox{GHz}}}}}}}.
Therefore, high-frequency electromagnetic waves have short wavelengths and high energy while low-frequency waves have long wavelengths and low energy.
In general, electromagnetic radiation is classified based on its wavelength as radio waves, microwaves, infrared, visible -which we perceive as visible light-, ultraviolet, X-rays and gamma rays.
The behavior of electromagnetic radiation depends on its wavelength. When electromagnetic radiation interacts with point atoms and molecules, its behavior also depends on the amount of energy per quantum it carries. Like sound waves, electromagnetic radiation can be divided into octaves.
Spectroscopy can detect a much broader region of the electromagnetic spectrum than the visible range of 400 to 700 nm. A typical laboratory spectrometer detects wavelengths from 2 to 2,500 nm.
Bands of the electromagnetic spectrum
For its study, the electromagnetic spectrum is divided into segments or bands, although this division is inaccurate. There are waves that have one frequency, but several uses, so some frequencies can sometimes be included in two ranges.
| Region | Wave length (m) | Frequency (Hz) | Energy (J) |
|---|---|---|---|
| Gamma rays | 10x10−12m | 30,0x1018Hz | ▪ 20·10−15 J |
| X-ray | 10x10−9m | 30,0x1015Hz | ▪ 20·10−18 J |
| Extreme ultraviolet | 200x10−9m | 1,5x1015Hz | ▪ 993·10−21 J |
| Near | 380x10−9m | 7,89x1014Hz | 523·10−21 J |
| Visible Spectrum | 780x10−9m | 384x1012Hz | 255·10−21 J |
| Near infrared | 2.5x10−6m | 120x1012Hz | ▪ 79·10−21 J |
| Infrared medium | 50x10−6m | 6,00x1012Hz | 4·10−21 J |
| Far/submillimetric infrared | 1x10−3m | 300x109Hz | ▪ 200·10−24 J |
| Microwave | . 10−2m | 3x108Hz | 2·10−24 J |
| Ultra High Frequency-Radio | 1 m | 300x106Hz | 한 19.8·10−26 J |
| Very High Frequency-Radio | 10 m | 30x106Hz | 한 19.8·10−28 J |
| Onda Corta - Radio | 180 m | 1,7x106Hz | ▪ 11.22·10−28 J |
| Onda Media - Radio | 650 m | 650x103Hz | ▪ 42.9·10−29 J |
| Onda Larga - Radio | 10x103m | 30x103Hz | 한 19.8·10−30 J |
| Very Low Frequency - Radio | 10x103m | 30x103Hz | 19.8·10−30 J |
Radio Frequency
In radio communications, ranges are abbreviated with their abbreviations in English. The ranges are:
Name English abbreviation ITU Banda Frequency Wave length Extremely low frequency TLF Not applicable Inferior to 3 Hz ▪ 100 000 km Extra low frequency ELF 1 3-30 Hz 100 000-10 000 km Super low frequency SLF 2 30-300 Hz 10 000-1000 km Ultra low frequency ULF 3 300-3000 Hz 1000-100 km Very low frequency VLF 4 3-30 kHz 100-10 km Low frequency LF 5 30-300 kHz 10-1 km Average frequency MF 6 300-3000 kHz 1 km-100 m High frequency HF 7 3-30 MHz 100-10 m Very high frequency VHF 8 30-300 MHz 10-1 m Ultra high frequency UHF 9 300-3000 MHz 1 m-100 mm Super high frequency SHF 10 3-30 GHz 100-10 mm High frequency EHF 11 30-300 GHz 10-1 mm Thinly high frequency THF 12? Over 300 GHz . 1 mm
- Extremely low frequency: Calls ELF (Extremely Low Frequencies), are those found in the interval of 3 to 30 Hz. This range is equivalent to those sound frequencies in the lowest (grease) part of the human ear perception interval. It should be noted here that the human ear perceives sound waves, not electromagnetic waves; however, the analogy is established to make a better comparison.
Minor frequencies have not been detected or until now (and maybe never) cannot be used (these are the TLFs)
- Super low frequency: SLF (Super Low Frequencies), are those found in the range of 30 to 300 Hz. This range includes electromagnetic waves of frequency equivalent to the serious sounds that the typical human ear perceives.
- Ultra low frequency: ULF (Ultra Low Frequencies), are those in the range of 300 to 3000 Hz. This is the interval equivalent to normal sound frequency for most of the human voice.
- Very low frequency: VLF (Very Low Frequencies). The frequencies of 3 to 30 kHz can be included here. The VLF interval is typically used in government and military communications.
- Low frequency: LF (Low Frequencies), are those in the range of 30 to 300 kHz. The main communications services that work in this range are aviation and marine navigation.
- Average frequency: MF (Medium Frequencies), are in the range of 300 to 3000 kHz. The most important waves in this range are the radio broadcasting of AM (530 to 1605 kHz).
- High frequency: HF (High Frequencies), are those contained in the range of 3 to 30 MHz. These are also known as "shortwave". It is at this interval that there is a wide range of types of radio communications such as radio broadcasting, government and military communications. Radio amateur and civil band communications also occur in this part of the spectrum.
- Very high frequency: VHF (Very High Frequencies), they go from 30 to 300 MHz. It is a popular range used for many services, such as mobile radio, marine and aeronautical communications, FM radio transmission (88 to 108 MHz) and TV channels from 2 to 12 [according to CCIR Standard (B+G Europe Standard)]. There are also several radio bands in this range.
- Ultra-high frequency: UHF (Ultra High Frequencies), range from 300 to 3000 MHz, includes UHF television channels, that is, from 21 to 69 [according to CCIR (B+G Europe Standard)] and are also used in mobile ground communication services, cell phone services and military communications.
- Super high frequencies: SHF (Super High Frequencies), are those between 3 and 30 GHz and are widely used for satellite communications and terrestrial radio links. In addition, they are intended to be used in very short-range high-rate data transmission communications using UWB. They are also used for military purposes, such as UWB-based radars.
- Extremely high frequency: EHF (Extremely High Frequencies), extend from 30 GHz to 300 GHz. The equipment used to transmit and receive these signals are more complex and costly, so they are not very diffused yet (the high speed 5g networks and the Internet of Things are also included).
- Thickly high frequencies: THF (Tremendously High Frequencies), extend from 300 GHz to more GHz. The equipment used to transmit and receive these signals to date are experimental.It could be used for short distance telecommunications.This would be the last usable frequency
There are other ways of sorting radiofrequency waves.
Microwave
The definition of the microwave spectrum depends on the source. Several authors consider microwaves to span frequencies between 300 MHz and 300 GHz, but IEC 60050 and IEEE 100 standards place the spectrum between 1 GHz and 300 GHz. These frequencies span part of the UHF range and the entire SHF range and ehf. These waves are used in numerous systems, such as multiple data transmission devices, radar, and microwave ovens.
| Banda | P | L | S | C | X | Ku | K | Ka | Q | U | V | E | W | F | D |
| Home (GHZ) | 0.2 | 1 | 2 | 4 | 8 | 12 | 18 | 26.5 | 30 | 40 | 50 | 60 | 75 | 90 | 110 |
| Final (GHZ) | 1 | 2 | 4 | 8 | 12 | 18 | 26.5 | 40 | 50 | 60 | 75 | 90 | 110 | 140 | 170 |
Infrared
Infrared waves are in the range of 0.7 to 1000 micrometers. Infrared radiation is generally associated with heat. They are produced by bodies that generate heat, although they can sometimes be generated by some light-emitting diodes and some lasers.
The signals are used for some special communication systems, such as in astronomy to detect stars and other bodies where heat detectors are used to discover moving bodies in the dark. They are also used in remote controls for televisions and other devices, in which a transmitter of these waves sends a coded signal to the television receiver. Lately, LAN local area connections have been implemented through devices that work with infrared, but due to new communication standards, these connections have lost their versatility.
Visible spectrum
| Color | Wave length |
|---|---|
| Morado | 380-450 nm |
| blue | 450-495 nm |
| Green | 495-570 nm |
| yellow | 570-590 nm |
| orange | 590-620 nm |
| Red | 620-750 nm |
Above the frequency of infrared radiation is what is commonly called light, a special type of electromagnetic radiation that has a wavelength in the range of 0.4 to 0.8 micrometers. This is the range in which the sun and similar stars emit most of their radiation. It is probably not a coincidence that the human eye is sensitive to the wavelengths that the sun emits most strongly. The usual units to express wavelengths are the Angstrom and the nanometer. The light that we see with our eyes is actually a very small part of the electromagnetic spectrum. Electromagnetic radiation with a wavelength between 380 nm and 760 nm (790-400 terahertz) is detected by the human eye and is perceived as visible light. Other wavelengths, especially near infrared (greater than 760 nm) and ultraviolet (less than 380 nm) are also sometimes referred to as light, even though visibility to humans is not relevant. If radiation having a frequency in the visible region of the electromagnetic spectrum is reflected from an object, for example a bowl of fruit, and then strikes the eyes, this gives rise to the visual perception of the scene. Our brain's visual system processes the multitude of frequencies that are reflected in different tones and hues, and through this not fully understood psychophysical phenomenon, most people perceive a bowl of fruit; a rainbow shows the optical (visible) electromagnetic spectrum. At most wavelengths, however, electromagnetic radiation is not directly visible, although technology exists capable of manipulating and visualizing a wide range of wavelengths.
Light can be used for different types of communications. Electromagnetic waves can be modulated and transmitted through optical fibers, resulting in less signal attenuation than free space transmission.
Ultraviolet
UV light covers the range from 4 to 400 nm. The Sun is an important emitting source of rays at this frequency, which cause skin cancer at prolonged exposures. This type of wave is not used in telecommunications, its applications are mainly in the field of medicine.
X-rays
The name X-rays designates an invisible electromagnetic radiation, capable of passing through opaque bodies and of impressing photographic films. The wavelength is between 10 and 0.01 nanometers, corresponding to frequencies in the range of 30 to 30,000 PHz (50 to 5,000 times the frequency of visible light).
Gamma Rays
Gamma radiation is a type of electromagnetic radiation produced generally by radioactive elements or subatomic processes such as the annihilation of a positron-electron pair. This type of radiation of such magnitude is also produced in astrophysical phenomena of great violence.
Due to their high energies, gamma rays are a type of ionizing radiation capable of penetrating matter more deeply than alpha or beta radiation. Given their high energy, they can cause serious damage to the nucleus of cells, which is why they are used to sterilize medical equipment and food.
Doppler effect
When the electromagnetic spectrum of light from a star or galaxy is analyzed, a redshift or a blueshift can be seen in it, that is, the visible colors move towards one end or the other of the visible spectrum. This occurs thanks to the Doppler effect, named after the Austrian physicist Christian Andreas Doppler, it is the apparent change in frequency of a wave produced by the relative movement of the source with respect to its observer. Doppler proposed this effect in 1842 in his treatise Über das farbige Licht der Doppelsterne und einige andere Gestirne des Himmels (On the color of light in binary stars and other bodies).
In the case of the visible spectrum of electromagnetic radiation, if the object moves away, its light shifts to longer wavelengths, redshifting. If the object gets closer, its light has a shorter wavelength, shifting towards blue. This shift towards red or blue is very slight even for high velocities, such as the relative velocities between stars or between galaxies, and cannot be captured by the human eye, only measured indirectly using precision instruments such as spectrometers. If the emitting object moved at significant fractions of the speed of light, the variation in wavelength would be directly appreciable.
The first Doppler redshift was described in 1848 by the French physicist Hippolyte Fizeau, who indicated that the shift in spectral lines seen in stars was due to the Doppler shift. In 1868, British astronomer William Huggins was the first to determine the speed of a star moving away from Earth using this method.
The abundance of redshift in the universe has allowed the creation of the theory of the expansion of the universe. The blue shift of the spectrum is observed in the Andromeda galaxy, which indicates that it is approaching, and in some galaxy arms, which allows us to discover its spin.
Contenido relacionado
Ether (physics)
Fermat's spiral
Astrodynamics