Electromagnetic radiation

Electromagnetic radiation is a type of variable electromagnetic field, that is, a combination of oscillating electric and magnetic fields, which propagate through space transporting energy from one place to another. From In the classical view, electromagnetic radiation is electromagnetic waves generated by sources of the electromagnetic field and propagating at the speed of light. The generation and propagation of these waves are compatible with the mathematical equation model defined in Maxwell's equations.
Electromagnetic radiation can manifest itself in many forms, including radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays. Unlike other types of waves, such as sound, which require a material medium to propagate, electromagnetic radiation can propagate in a vacuum. In the 19th century it was thought that there was an undetectable substance called ether, which occupied a vacuum and served as a medium for the propagation of electromagnetic waves. The theoretical study of electromagnetic radiation is called electrodynamics and is a subfield of electromagnetism.
Electromagnetic waves can be generated by different sources such as: accelerated charges, oscillating dipoles, variable currents in different types of antennas, among others. The shape of electromagnetic waves depends on the source that generates them and the distance traveled by them.
Story of discovery
Electromagnetic radiation of wavelengths other than visible light was discovered in the early 19th century. The discovery of infrared radiation is attributed to astronomer William Herschel, who published his results in 1800 before the Royal Society of London. Herschel used a glass prism to refract sunlight and detected invisible rays that caused heating beyond the red part of the spectrum, by an increase in temperature recorded with a thermometer. These "heat rays" were later called infrared.
In 1801, German physicist Johann Wilhelm Ritter discovered ultraviolet light in an experiment similar to Herschel's, using sunlight and a glass prism. Ritter noted that invisible rays near the violet edge of a solar spectrum scattered by a triangular prism darkened silver chloride preparations more rapidly than violet light. Ritter's experiments were the first forerunners of what would become photography. Ritter noted that ultraviolet rays (at first called "chemical rays") were capable of causing chemical reactions.
In 1862-1864, James Clerk Maxwell developed equations for the electromagnetic field that suggested that waves in the field would travel very close to the speed of light. Thus, Maxwell suggested that visible light (as well as inferentially invisible infrared and ultraviolet rays) consisted of propagating disturbances (or radiation) in the electromagnetic field. Radio waves were first deliberately produced by Heinrich Hertz in 1887, using electrical circuits calculated to produce oscillations at a frequency much lower than that of visible light. Hertz also developed ways to detect these waves and produced and characterized what were later called radio waves and microwaves.
Wilhelm Röntgen discovered and named x-rays. After experimenting with high voltages applied to a vacuum tube on November 8, 1895, he noticed fluorescence on a nearby plate of coated glass. In one month, he discovered the main properties of X-rays.
The last part of the electromagnetic spectrum to be discovered was associated with radioactivity. Henri Becquerel discovered that uranium salts caused an unexposed photographic plate to cloud through a cover paper in a manner similar to X-rays, and Marie Curie discovered that only certain elements emitted these rays of energy, soon discovering the intense radium radiation. Pitchblende radiation was differentiated into alpha rays (alpha particles) and beta rays (beta particles) by Ernest Rutherford through simple experimentation in 1899, but these were shown to be charged particle types of radiation. However, in 1900, the French scientist Paul Villard discovered a third type of neutrally charged and especially penetrating radium radiation, and after describing it, Rutherford realized that it must be a third type of radiation, which in 1903 he called rays. gamma. In 1910, the British physicist William Henry Bragg showed that gamma rays are electromagnetic radiation, not particles, and in 1914 Rutherford and Edward Andrade measured their wavelengths, finding them to be similar to X-rays but with shorter wavelengths and higher frequency.
Phenomena associated with electromagnetic radiation
There are many physical phenomena associated with electromagnetic radiation that can be studied in a unified way, such as the interaction of electromagnetic waves and charged particles present in matter. Among these phenomena are, for example, visible light, radiated heat, radio and television waves or certain types of radioactivity to name some of the most outstanding phenomena. All these phenomena consist of the emission of electromagnetic radiation in different frequency ranges (or equivalently different wavelengths), the frequency range or wavelength being the most used to classify the different types of electromagnetic radiation. The arrangement of the various types of electromagnetic radiation by frequency is called the electromagnetic spectrum.
Visible light
Visible light is made up of electromagnetic radiation whose wavelengths are between 400 and 700 nm. Light is produced in the atomic shell of atoms, when an atom receives energy for various reasons, some of its electrons may move to higher-energy electronic layers. Electrons are unstable in higher energy layers if there are unoccupied lower energy levels, so they tend to fall towards these, but when they decay towards lower levels, energy conservation requires the emission of photons, whose frequencies usually fall in the ranges associated with visible light. That is precisely what happens in phenomena of primary emission as diverse as the flame of fire, an incandescent filament of a lamp or light from the sun. Secondarily, the light coming from primary emission can be partially reflected, refracted, absorbed and that is the reason why objects that are not primary emission sources are visible.
Thermal radiation
When metal and other substances are subjected to heat sources, they heat up and emit visible light. For a metal this phenomenon is called heating "red hot", since the light initially emitted is reddish-orange, if the temperature rises more white-yellowish. It should be noted that before the light emitted by metals and other superheated substances is visible, these same bodies radiate heat in the form of infrared radiation, which is a type of electromagnetic radiation that is not directly visible to the human eye.
Interaction between electromagnetic radiation and conductors
When a wire or any conductive object, such as an antenna, conducts alternating current, electromagnetic radiation propagates at the same frequency as the current.
Similarly, when electromagnetic radiation falls on an electrical conductor, it causes the electrons on its surface to oscillate, thus generating an alternating current whose frequency is the same as that of the radiation incident. This effect is used in antennas, which can act as emitters or receivers of electromagnetic radiation.
Studies by analysis of the electromagnetic spectrum
A lot of information about the physical properties of an object can be obtained by studying its electromagnetic spectrum, either from light emitted (blackbody radiation) or absorbed by it. This is spectroscopy and it is widely used in astrophysics and chemistry. For example, hydrogen atoms have a natural frequency of oscillation, so they emit radio waves, which have a wavelength of 21.12 cm.
Penetration of electromagnetic radiation
Depending on the frequency, electromagnetic waves may not pass through conductive media. This is the reason why radio transmissions do not work under the sea and mobile phones are left without coverage inside a metal box. However, since energy is neither created nor destroyed, when an electromagnetic wave collides with a conductor, two things can happen. The first is that they are transformed into heat: this effect has an application in microwave ovens. The second is that they are reflected from the conductor's surface (as in a mirror).
Refraction
The speed of propagation of electromagnetic radiation in a vacuum is «c». Electromagnetic theory states that:
- c=1ε ε 0μ μ 0{displaystyle c={frac {1}{sqrt {varepsilon _{0}mu _{0}}}}}}}{varepsilon
where ε ε 0{displaystyle varepsilon} and μ μ 0{displaystyle mu _{0}} are the electric permitivity and magnetic permeability of the vacuum respectively.
In a material medium electric permitivity ε ε {displaystyle varepsilon } has a different value ε ε 0{displaystyle varepsilon}. The same occurs with magnetic permeability μ μ {displaystyle mu } and, therefore, the speed of light in that medium v{displaystyle v} will be different from c. The speed of propagation of light in different means to the vacuum is always lower than c.
When light changes from one medium to another, it experiences a deflection that depends on the angle at which it hits the surface that separates the two media. One speaks, then, of an incident angle and a transmission angle. This phenomenon, called refraction, is clearly noticeable in the deviation that light beams show when they hit the water. The speed of light in a medium can be calculated from its electrical permittivity and its magnetic permeability as follows:
- v=1ε ε μ μ {displaystyle v={frac {1}{sqrt {varepsilon mu }}}}}}
Scatter
The electrical permittivity and magnetic permeability of a medium other than a vacuum depend, in addition to the nature of the medium, on the wavelength of the radiation. From this it follows that the speed of propagation of electromagnetic radiation in a medium also depends on the wavelength of said radiation. Therefore, the deflection of a ray of light when changing medium will be different for each color (for each wavelength). The clearest example is that of a beam of white light that "decomposes" in colors when passing through a prism. White light is really the sum of light beams of different wavelengths, which are bent in different ways. This phenomenon is called dispersion. It is the cause of chromatic aberration, the halo of colors that can be seen around objects when observing them with instruments that use lenses such as binoculars or telescopes.
Radiation by accelerated particles
An important consequence of classical electrodynamics is that a charged particle in accelerated motion (rectilinear, circular or of another type) must emit electromagnetic waves, the emitted power being proportional to the square of its acceleration, in fact Larmor's formula for the emitted power is given by:
P=q2a26π π ε ε 0c3{displaystyle P={frac {q^{2}a^{2}}{6pi varepsilon _{0}{text{c}}{3}}}}}{3}}}}}}}
Where:
- q{displaystyle q,} is the electrical load of the particle.
- a{displaystyle a,} is the acceleration of the particle.
- ε ε 0{displaystyle varepsilon _{0},} the electric permitivity of the vacuum.
- c{displaystyle {text{c}},} It's the speed of light.
An example of this phenomenon of radiation emission by charged particles is synchrotron radiation.
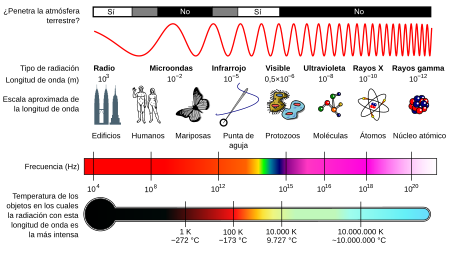
Electromagnetic spectrum
According to its wavelength, electromagnetic radiation receives different names, and varies from energetic gamma rays (with a wavelength of the order of picometers) to radio waves (wavelengths of the order of kilometers), going through the visible spectrum (whose wavelength is in the range of tenths of a micrometer). The entire range of wavelengths is called the electromagnetic spectrum.
The visible spectrum is a tiny range from the violet wavelength (approximately 400 nanometers) to the red wavelength (approximately 700 nm).
In telecommunications, waves are classified by an international frequency agreement based on the use for which they are intended as shown in the table, in addition a special type called microwaves must be considered, whose frequency range is between 1 GHz and 300 GHz, that is, wavelengths between 30 centimeters and 1 millimeter, which have the capacity to cross the terrestrial ionosphere, allowing satellite communication.
| Acronym | Rank | Denomination | Employment |
|---|---|---|---|
| VLF | 10 kHz to 30 kHz | Very low frequency | Radio outreach |
| LF | 30 kHz to 300 kHz | Low frequency | Radio, navigation |
| MF | 300 kHz to 3 MHz | Average frequency | Average wave radio |
| HF | 3 MHz to 30 MHz | High frequency | Shortwave radio |
| VHF | 30 MHz to 300 MHz | Very high frequency | TV, radio |
| UHF | 300 MHz to 3 GHz | Ultra high frequency | TV, radar, mobile phone,
WLAN |
| SHF | 3 GHz to 30 GHz | Super high frequency | Radar |
| EHF | 30 GHz to 300 GHz | Extremely high frequency | Radar |
Theoretical explanations of electromagnetic radiation
Classical electromagnetism and quantum mechanics offer different descriptions of electromagnetic radiation. In classical electromagnetism radiation is an oscillating field that propagates from the emitting source, while in quantum mechanics radiation is interpreted in terms of particles (photons) emitted by a source. These two descriptions, however, are complementary and for macroscopic situations they are not qualitatively different.
Maxwell's Equations
Maxwell associated several equations, currently called Maxwell's equations, from which it follows that an electric field that varies in time generates a magnetic field and, reciprocally, the temporal variation of the magnetic field generates a electric field. It can be verified that this "induction" mutual makes Maxwell's equations admit of a solution in the form of a wave propagating from a source. This theoretical solution was the one that led to postulate that electromagnetic waves and electromagnetic radiation would exist, and even to postulate that light itself was an electromagnetic wave.
Electromagnetic radiation can be visualized as two fields that are generated mutually, so they do not need any material means to propagate. Maxwell's equations also predict the speed of propagation in a vacuum (represented c, by the speed of light, with a value of 299,792,458 m/s), and its direction of propagation (perpendicular to the oscillations of the electric and magnetic field which, in turn, are perpendicular to each other).
Wave-particle duality
Depending on the phenomenon studied, electromagnetic radiation can be considered not as a series of waves but as a beam or flow of particles, called photons. This wave-particle duality means that each photon has an energy directly proportional to the frequency of the associated wave, given by Planck's relation:
E=h.. {displaystyle E=hnu ,}
where E{displaystyle E} is the energy of the photon, h{displaystyle h} It's Planck's constant and .. {displaystyle nu } It's the frequency of the wave.
Value of Planck's constant
- h=6,6260693(11)× × 10− − 34J⋅ ⋅ s=4,13566743(35)× × 10− − 15eV⋅ ⋅ s{displaystyle h=,,,6,626 0693(11)times 10^{-34} {mbox{J}}{cdot {mbox{s}},,,=,,,4,135 667times 10^{-15}{mbox{eV}{cdot {cdot {}{
Also, considering electromagnetic radiation as wave, wavelength λ λ {displaystyle lambda } and oscillation frequency .. {displaystyle nu } are related by a constant, the speed of light in the middle (c in the void):
c=λ λ .. {displaystyle {text{c}}=lambda nu ,}
The longer the wavelength, the lower the frequency (and the lower the energy according to Planck's relation).
An example of an electromagnetic wave would be that of the radio since the signal propagates through it until it reaches the receiver, which in this case would be the radio since it propagates through space transporting energy from one place to another.
Biological effects
Bioelectromagnetism is the study of the interactions and effects of electromagnetic radiation on living organisms. The effects of electromagnetic radiation on living cells, including humans, depend on the power and frequency of the radiation. In the case of low-frequency radiation (radio waves to visible light), the best understood effects are those due solely to the power of the radiation, acting through heating when the radiation is absorbed. For these thermal effects, the frequency is important as it affects the intensity of the radiation and the penetration into the organism (for example, microwaves penetrate better than infrared). It is widely accepted that low-frequency fields that are too weak to cause significant warming could not have any biological effect.
Despite the commonly accepted results, some research has been done to show that weaker non-thermal electromagnetic fields, and modulated radiofrequency and microwave fields have biological effects. The fundamental mechanisms of the interaction between the Biological material and electromagnetic fields at non-thermal levels are not fully understood.
The World Health Organization has classified radiofrequency electromagnetic radiation in Group 2B, possibly carcinogenic. This group contains possible carcinogens such as lead, DDT, and styrene. For example, epidemiological studies looking for a relationship between cell phone use and the development of brain cancer have been largely inconclusive, except to show that the effect, if it exists, cannot be large.
At higher frequencies (visible and beyond), the effects of individual photons begin to become important, as these now individually have enough energy to directly or indirectly damage biological molecules. All ultraviolet frequencies have been classified as a Group 1 carcinogen by the World Health Organization. Ultraviolet radiation from sun exposure is the leading cause of skin cancer.
Thus, at ultraviolet and higher frequencies, electromagnetic radiation causes more damage to biological systems than simple heating would predict. Ultraviolet rays, along with X-rays and gamma radiation, are called ionizing radiation because of the ability of photons from this radiation to produce ions and free radicals in materials (including living tissue). Because such radiation can severely harm life at energy levels that produce little heating, it is considered much more dangerous (in terms of damage done per unit of energy or power) than the rest of the electromagnetic spectrum.
Use as a weapon
The heat beam is an application of electromagnetic energy that uses microwave frequencies to create an unpleasant heating effect on the top layer of the skin. The US military developed a publicly known heat ray weapon called the Active Denial System as an experimental weapon to deny enemy access to an area. A death ray is a theoretical weapon that emits a heat beam based on electromagnetic energy at levels that are capable of damaging human tissue. One inventor of a death ray, Harry Grindell Matthews, claimed to have lost sight in his left eye while working on his microwave magnetron-based death ray weapon from the 1920s.
Contenido relacionado
Colloid
Degree Fahrenheit
Gram