Distillation
distillation is the process of separating the components or substances of a liquid mixture through the use of selective boiling and condensation. Distillation can result in essentially complete separation (nearly pure components), or it can be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the volatility of the mixture components. In industrial chemistry, distillation is a unit operation of virtually universal importance, but it is a process of physical separation; not a chemical reaction.
Distillation has many applications. For example:
- The distillation of fermented products produces distilled beverages with a high alcohol content or separates other fermentation products of commercial value.
- Distillation is an effective and traditional method of desalination.
- In the fossil fuel industry, oil stabilization is a form of partial distillation that reduces steam pressure from crude oil, making it safe for storage and transportation, as well as reducing emissions to the atmosphere of volatile hydrocarbons. In intermediate operations in oil refineries, distillation is an important class of operations to transform crude oil into chemical fuels and raw materials.
- Cryogenic distillation leads to the separation of air into its components, especially oxygen, nitrogen and argon, for industrial use.
- In the field of industrial chemistry, large quantities of raw chemical synthesis liquid products are distilled to separate them, either from other products, from impurities or from starting materials without reacting.
A facility used for distillation, especially distilled spirits, is known as a distillery. The distillation equipment in a distillery is a still.
History
In 1975, Paolo Rovesti (1902-1983), a chemist and pharmacist known as the "father of phytocosmetics", discovered a terracotta distillation apparatus in the Indus Valley, West Pakistan, dating to around 3000 BCE. Early evidence of distillation was found on Akkadian tablets dated year 1200 BC that describe perfumery operations. The tablets provided textual evidence that the Babylonians of ancient Mesopotamia knew of a primitive form of distillation. Early evidence of distillation was also found relating to alchemists working in Alexandria in Roman Egypt in the I. Distilled water has been in use since at least 200 AD. C., when Alexander of Aphrodisias described the process. Work to distill other liquids continued in ancient Byzantine Egypt under Zosimus of Panopolis in the III. Distillation was practiced on the ancient Indian subcontinent which is evident in fired clay retorts and receivers found at Taxila and Charsadda in modern Pakistan, dating back to the early centuries CE. These "stills from [Gandhara]" they could only produce very weak liquor, as there was no efficient means of collecting the simmering vapors. Distillation in China may have begun during the Eastern Han dynasty (2nd-2nd century), but distillation of beverages began in the Jin (12th-13th century) and Southern Song (12th-13th century) dynasties, based on archaeological evidence.
Clear evidence for the distillation of alcohol comes from the Arab chemist Al-Kindi in 9th century IraqIX. The process later spread to Italy, where it was described by the Salerno School in the 12th century. Fractional distillation was developed by Tadeo Alderotti in the 13th century. A still was found at an archaeological site in Qinglong, Hebei province, China, dating from the 12th century century. Distilled spirits were common during the Yuan Dynasty (13th-14th century).
In 1500, the German alchemist Hieronymus Braunschweig published Liber de arte destillandi (The Book of the Art of Distillation), the first book devoted exclusively to the subject of distillation. distillation, followed in 1512 by a much enlarged version. In 1651, John French published The Art of Distillation the first major English-language compendium of the practice, but it has been claimed that much of it derives from Braunschweig's work. This includes diagrams with people showing the industrial scale rather than the scale of the operation.
As alchemy evolved into the science of chemistry, vessels called retorts were used for distillation. Both stills and retorts are forms of glassware with long necks that point to the side at a downward angle to act as air-cooled condensers to condense the distillate and allow it to trickle down for collection. Subsequently, copper stills were invented. Riveted joints were often held tight using various mixtures, for example, a dough made from rye flour. These stills often featured a cooling system around the spout, using cold water, for example, which made alcohol condensation more efficient. These were called kettles. Today, replicas and stills have been largely supplanted by more efficient distillation methods in most industrial processes. However, the kettle is still widely used for the production of some fine spirits, such as cognac, Scotch whiskey, Irish whiskey, tequila and some vodkas. Smugglers of various materials (wood, clay, stainless steel) are also used by smugglers in various countries. Small stills are also sold for use in home production of flower water or essential oils.
Early forms of distillation involved batch processes using vaporization and condensation. Purity was improved by further distillation of the condensate. The largest volumes were processed simply by repeating the distillation. Chemists reportedly performed between 500 and 600 distillations to obtain a pure compound.
In the early 19th century century, the basics of modern techniques were developed, including preheating and reflow. In 1822, Anthony Perrier developed one of the first continuous stills, and then, in 1826, Robert Stein improved on that design to make his distillation column. In 1830, Aeneas Coffey obtained a patent to further improve the design. Coffey's continuous still can be considered the archetype of modern petrochemical units. French engineer Armand Savalle developed his steam regulator around 1846. In 1877 Ernest Solvay was granted a US patent for a pan column for the distillation of ammonia and in the same year and in the years Subsequent advances were made on this issue for oils and spirits.
With the rise of chemical engineering as a discipline in the late 19th century, scientific rather than empirical methods could be applied. The development of the oil industry at the turn of the 20th century provided the impetus for the development of precise design methods, such as the method Ernest Thiele's McCabe-Thiele and the Fenske equation. The availability of powerful computers also allowed direct simulations of distillation columns.
Distillation applications
The application of distillation can be roughly divided into four groups:
- laboratory scale,
- industrial distillation,
- distillation of herbs for perfumery and medications (weedless) and
- food processing.
The last two are distinctively different from the previous two in that distillation is not used as a true purification method, but rather to transfer all the volatiles from the source materials to the distillate in beverage and herb processing.
The main difference between lab-scale distillation and industrial distillation is that lab-scale distillation often occurs in a batch manner, while industrial distillation often occurs continuously. In batch distillation, the composition of the source material, the vapors of distillation compounds, and the distillate change during distillation. In batch distillation, a still is charged (supplied) with a batch of feed mixture, which is then separated into its component fractions, which are collected sequentially from most volatile to least volatile, with bottoms, fraction minimal or non-volatile remaining. removed at the end. The still can be recharged and the process repeated.
In continuous distillation, the source materials, vapors, and distillate are kept at a constant composition by carefully topping up the source material and removing both vapor and liquid fractions from the system. This results in more detailed control of the separation process.
Idealized distillation model
The boiling point of a liquid is the temperature at which the vapor pressure of the liquid equals the pressure around the liquid, allowing bubbles to form without being crushed. A special case is the normal boiling point, where the vapor pressure of the liquid is equal to the ambient atmospheric pressure.
It is a common misconception that in a liquid mixture at a given pressure, each component boils at the boiling point corresponding to the given pressure, allowing the vapors of each component to accumulate separately and purely. However, this does not occur, even in an idealized system. Idealized distillation models are essentially governed by Raoult's law and Dalton's law and assume that vapor-liquid equilibria are reached.
Raoult's law states that the vapor pressure of a solution depends on
- steam pressure of each chemical component in the solution and
- the solution fraction that each component constitutes, also known as the molar fraction.
This law applies to ideal solutions, or solutions that have different components but whose molecular interactions are the same or very similar to pure solutions.
Dalton's law states that the total pressure is the sum of the partial pressures of each individual component in the mixture. When a multi-component liquid is heated, the vapor pressure of each component will increase, causing the total vapor pressure to increase. When the total vapor pressure reaches the pressure surrounding the liquid, boiling occurs and the liquid becomes a gas throughout the volume of the liquid. Note that a mixture with a given composition has a boiling point at a given pressure when the components are mutually soluble. A mixture of constant composition does not have multiple boiling points.
One implication of a boiling point is that the lightest components never "boil first" in a clean way. At boiling point, all volatile components boil, but for one component, its percentage in the vapor is the same as its percentage of the total vapor pressure. The lighter components have a higher partial pressure and therefore concentrate in the vapor, but the heavier volatile components also have a (smaller) partial pressure and also necessarily vaporize, albeit at a lower concentration in the vapor. steam. In fact, batch and fractional distillation succeed by varying the composition of the mixture. In batch distillation, the batch is vaporized, which changes its composition; in fractionation, the liquid higher in the fractionation column contains more lights and boils at lower temperatures. Thus, from a given mixture, it appears to have a boiling range rather than a boiling point, although this is because its composition changes: each mixture in between has its own, singular boiling point.
The idealized model is accurate for chemically similar liquids, such as benzene and toluene. In other cases, serious deviations from Raoult's law and Dalton's law are observed, especially in the mixture of ethanol and water. These compounds, when heated together, form an azeotrope, which is when the vapor phase and the liquid phase contain the same composition. Although there are computational methods that can be used to estimate the behavior of a mixture of arbitrary components, the only way to obtain accurate liquid-vapor equilibrium data is by measurement.
It is not possible to completely purify a mixture of components by distillation, as this would require each component in the mixture to have a partial pressure of zero. If ultra-pure products are the goal, additional chemical separation must be applied. When a binary mixture is vaporized and the other component, say a salt, has a partial pressure of zero for practical purposes, the process is simpler.
Batch or differential distillation
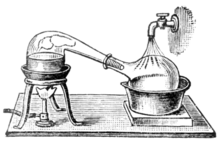
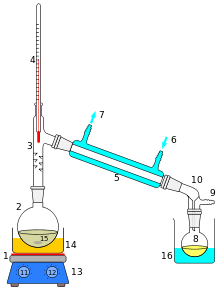
Heating an ideal mixture of two volatile substances, A and B, with A having the higher volatility, or lower boiling point, in a batch distillation setup (as in an apparatus depicted in the opening figure) until the mixture boils, resulting in a vapor over the liquid containing a mixture of A and B. The ratio between A and B in the vapor will be different from the ratio in the liquid. The ratio in the liquid will be determined by how the original mixture was prepared, while the ratio in the vapor will be enriched in the more volatile compound, A (due to Raoult's Law). The vapor passes through the condenser and is removed from the system. This, in turn, means that the proportion of compounds in the remaining liquid is now different from the initial proportion (ie, more B-enriched than in the starting liquid).
The result is that the ratio in the liquid mixture is changing and it is getting richer in component B. This causes the boiling point of the mixture to rise, resulting in an increase in temperature in the vapor, resulting in a changing ratio of A:B in the gas phase (as distillation continues, there is an increasing proportion of B in the gas phase). This results in a slowly changing A:B ratio in the distillate.
If the difference in vapor pressure between the two components A and B is large, usually expressed as the difference in boiling points, the mixture at the beginning of the distillation is highly enriched in component A, and when the component A is distilled, the boiling liquid is enriched in component B.
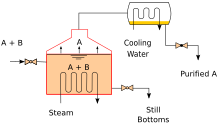
Continuous distillation
Continuous distillation is a distillation in which a liquid mixture is fed continuously (without interruption) into the process and the separated fractions are continuously removed as output streams occur over time during operation. Continuous distillation produces a minimum of two output fractions, including at least one volatile distillate fraction, which has been separately boiled and captured as a vapor and then condensed to a liquid. There is always a bottom fraction (or residue), which is the less volatile residue that has not been separately captured as condensed vapor.
Continuous distillation differs from batch distillation in that the concentrations must not change over time. Continuous distillation can be run in a steady state for an arbitrary period of time. For any source of specific composition, the main variables affecting the purity of the products in continuous distillation are the reflux ratio and the number of theoretical equilibrium stages, determined in practice by the number of plates or the height of the packing. Reflux is a flow from the condenser that returns to the column, generating a recycle that allows a better separation with a given number of plates. Equilibrium stages are ideal steps where the compositions achieve vapor-liquid equilibrium, repeating the separation process and allowing for better separation given a reflux ratio. A column with a high reflux ratio may have fewer stages, but a large amount of liquid refluxes, giving a broad column with a large diameter. Conversely, a column with a low reflux index must have a large number of stages, which requires a taller column.
General improvements
Both batch and continuous distillations can be improved by making use of a fractionating column at the top of the distillation flask. The column improves separation by providing a larger surface area for the steam and condensate to come into contact with. This helps you stay balanced for as long as possible. The column may even consist of small subsystems ('plates' or 'dishes') containing a boiling, enriched liquid mixture, all with their own vapor-liquid equilibrium.
There are differences between laboratory scale and industrial scale fractionation columns, but the principles are the same. Examples of laboratory scale fractionation columns (to increase efficiency) include
- Air Condenser
- Vigreux Column (usually lab scale only)
- Cold column (packed with glass beads, metal parts or other chemically inert material)
- Rotating band distillation system.
Distillation on a laboratory scale
Laboratory-scale distillations are performed almost exclusively as batch distillations. The device used in distillation, sometimes referred to as a still, consists of a minimum of a reboiler or kettle in which the source material is heated, a condenser in which the heated vapor is cooled back to the liquid state, and a receiver in which the concentrated or purified liquid, called the distilled, it is collected. Various laboratory-scale techniques exist for distillation (see also types of distillation).
Simple distillation
In simple distillation, the vapor is immediately channeled to a condenser. Consequently, the distillate is not pure, but its composition is identical to the composition of the vapors at the given temperature and pressure. This concentration follows Raoult's law.
As a result, simple distillation is effective only when the boiling points of the liquid differ greatly (the rule of thumb is 25 °C) or when separating liquids from non-volatile solids or oils. For these cases, the vapor pressures of the components are usually sufficiently different that the distillate can be pure enough for its intended purpose.
Fractional Distillation
In many cases, the boiling points of the components in the mixture will be close enough for Raoult's law to be taken into account. Therefore, fractional distillation should be used to separate the components by repeated vaporization-condensation cycles within a packed fractionating column. This separation, by successive distillations, is also known as rectification.
As the solution to be purified is heated, its vapors rise to the fractionation column. As it rises, it cools, condensing on the walls of the condenser and on the surfaces of the packing material. Here, the condensate continues to be heated by the rising vapors; it vaporizes once more. However, the composition of fresh vapors is once again determined by Raoult's law. Each vaporization-condensation cycle (called a theoretical plate) will produce a purer solution of the more volatile component. In reality, each cycle at a given temperature does not occur at exactly the same position in the fractionation column.; the theoretical plate is thus a concept rather than a precise description.
More theoretical plates lead to better separations. A rotating band distillation system uses a rotating Teflon or metal band to force rising vapors into close contact with descending condensate, increasing the number of theoretical plates.
Steam stripping distillation
Like vacuum distillation, steam stripping is a method of distilling compounds that are sensitive to heat. The temperature of the steam is easier to control than the surface of the a heating element and allows a high rate of heat transfer without heating to a very high temperature. This process involves bubbling steam through a hot mixture of the raw material. According to Raoult's law, part of the target compound will vaporize (according to its partial pressure). The vapor mixture cools and condenses, usually producing a layer of oil and a layer of water.
Steam distillation of various aromatic herbs and flowers can result in two products; an essential oil, as well as an aqueous herbal distillate. Essential oils are often used in perfumery and aromatherapy, while aqueous distillates have many applications in aromatherapy, food processing, and skin care.
Vacuum distillation
Some compounds have very high boiling points. To boil such compounds, it is often better to lower the pressure at which such compounds are boiled rather than increase the temperature. Once the pressure is reduced to the vapor pressure of the compound (at the given temperature), boiling and the rest of the distillation process can begin. This technique is known as vacuum distillation and is commonly found in the laboratory in the form of a rotary evaporator.
This technique is also very useful for compounds that boil past their decomposition temperature at atmospheric pressure and would therefore decompose by any attempt to boil them under atmospheric pressure.
Molecular distillation is vacuum distillation below the pressure of 0.01 torr. 0.01 torr is an order of magnitude above high vacuum, where the fluids are in the free molecular flow regime, ie the mean free path of the molecules is comparable to the size of the equipment. The gas phase no longer exerts significant pressure on the substance to be evaporated, and consequently, the rate of evaporation is no longer dependent on pressure. That is, because the continuous assumptions of fluid dynamics no longer apply, mass transport is governed by molecular dynamics rather than fluid dynamics. Therefore, a short path between the hot surface and the cold surface is necessary, typically by suspending a hot plate covered with feed film next to a cold plate with a line of sight in between. Molecular distillation is used industrially for the purification of oils.
Air Sensitive Vacuum Distillation
Some compounds have high boiling points as well as being sensitive to air. A simple vacuum distillation system can be used as exemplified above, whereby the vacuum is replaced with an inert gas after the distillation is complete. However, this is a less satisfactory system if one wishes to collect fractions under reduced pressure. To do this, a "cow" or "pig" at the condenser end, or for best results or for very air sensitive compounds, a Perkin triangle apparatus may be used.
The Perkin triangle has means through a series of glass or Teflon taps to allow the fractions to be isolated from the rest of the still, without the main body of the distillation being drawn from the vacuum or heat source, and thus can remain in a state of reflux. To do this, the sample is first isolated from the vacuum by means of the taps, then the vacuum on the sample is replaced with an inert gas (such as nitrogen or argon) and can then be capped and disposed of. A new collection vessel can then be added to the system, evacuated, and reconnected to the distillation system through the taps to collect a second fraction, and so on, until all fractions have been collected.
Short path distillation
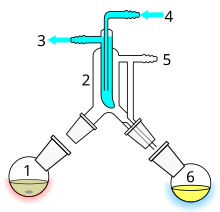
Short path distillation is a distillation technique that involves the distillate traveling a short distance, often only a few centimeters, and is usually done under reduced pressure. A classic example would be a distillation involving the distillate that travels from one glass bulb to another, without the need for a condenser to separate the two chambers. This technique is often used for compounds that are unstable at high temperatures or to purify small amounts of compound. The advantage is that the heating temperature can be considerably lower (at reduced pressure) than the boiling point of the liquid at standard pressure, and the distillate only has to travel a short distance before condensing. A short path ensures that little compound is lost on the sides of the apparatus. The Kugelrohr is a kind of short path distillation apparatus often containing multiple chambers to collect the distillate fractions.
Zone Distillation
Zone distillation is a long vessel distillation process with partial melting of refined matter in the moving liquid zone and condensation of vapor in the solid phase by dumping the condensate in a cold area. When the zone heater is moving from the top to the bottom of the container, a solid condensate is forming with an irregular impurity distribution. Then the purest part of the condensate can be extracted as a product. The process can be repeated many times by moving (without rotation) the received condensate to the bottom of the container at the place of the refined matter. The irregular distribution of impurities in the condensate (ie, the purification efficiency) increases with the number of repetitions of the process. Zone distillation is a distillation analogue of zone recrystallization. The distribution of impurities in the condensate is described by known zone recrystallization equations with various process iteration numbers, with an efficient replacement distribution of crystallization at the separation factor α from the distillation.
Other types
- The process of reactive distillation involves using the reaction vessel as an alambique. In this process, the product usually has a lower boiling point than its reagents. As the product is formed from the reactants, it is vaporized and removed from the reaction mix. This technique is an example of a continuous process against a batch process; the advantages include less inactivity time to load the reaction vessel with starting material and less work. The distillation "on a reactive" could be classified as a reactive distillation. It is usually used to remove volatile impurities from distalation feeding. For example, a bit of lime can be added to eliminate carbon dioxide from water, followed by a second distillation with a little sulfuric acid to remove the remains of ammonia.
- Catalytic distillation is the process by which the reagents are catalyzed while distillating to continuously separate the products from the reagents. This method is used to help balance reactions complete.
- Steaming is a method for the separation of fluid mixtures by partial vaporization through a non-porous membrane.
- Extractive distillation is defined as distillation in the presence of a miscible, high boiling point and relatively non volatile, solvent, which does not form azeotropo with the other components in the mixture.
- Instant evaporation (or partial evaporation) is the partial vaporization that occurs when a saturated fluid current suffers a reduction in pressure by passing through a strangulation valve or other strangulation device. This process is one of the simplest unit operations, being equivalent to a distillation with a single stage of balance.
- Codestilation is a distillation that is done in mixtures in which the two compounds are not miscible. In the laboratory, the Dean-Stark device is used for this purpose to remove the water from synthesis products. The Bleidner is another example with two reflux solvents.
- Membrane distillation is a type of distillation in which the vapors of a mixture to separate are passed through a membrane, which selectively permeates a component of the mixture. The steam pressure difference is the driving force. It has potential applications in the desalination of sea water and the elimination of organic and inorganic components.
The unit process of evaporation can also be called "distillation":
- A vacuum distillation device is used in rotating evaporation to remove the bulk solvents from a sample. Typically, the vacuum is generated by a water vacuum cleaner or a membrane pump.
- In a kugelrohr is typically used a short-haul distillation device (usually in combination with a (high) empty) to distill high boiling point (105 °C) compounds. The device consists of an oven in which the compound is placed to distill, a reception part that is outside the oven and a means of rotation of the sample. The vacuum is usually generated using a high vacuum pump.
Other uses:
- Dry distillation or destructive distillation in spite of the name, is not really distillation, but a chemical reaction known as pyrolysis in which solid substances are heated in an inert or reducing atmosphere and any volatile fraction, containing high boiling fluids and pyrolysis products. They pick up. The destructive distillation of wood to produce methanel is the root of its common name: wood alcohol.
- Frost distillation is an analogous method of purification that uses freezing instead of evaporation. It is not really a distillation, but a recristalization in which the product is the mother liquor and does not produce products equivalent to distillation. This process is used in the production of frost beer and ice wine to increase the content of ethanol and sugar, respectively. It is also used to produce applejack. Unlike distillation, frost distillation concentrates poisonous congeners rather than removing them; as a result, many countries prohibit applejack as a health measure. However, reducing methanel with the absorption of molecular sieve 4A is a practical method for production. In addition, evaporation distillation can separate them as they have different boiling points.
Azeotropic distillation
The interactions between the solution components create unique properties for the solution, since most processes involve non-ideal mixtures, where Raoult's law does not hold. Such interactions can result in a constant-boiling azeotrope that behaves as if it were a pure compound (ie, it boils at a single temperature rather than a range). In an azeotrope, the solution contains the given component in the same proportion as the vapor, so evaporation does not change the purity and distillation does not effect the separation. For example, ethyl alcohol and water form a 95.6% azeotrope at 78.1 °C.
If the azeotrope is not considered pure enough for use, there are some techniques to break the azeotrope to give a pure distillate. This set of techniques is known as azeotropic distillation. Some techniques accomplish this by "jumping" on the azeotrope composition (by adding another component to create a new azeotrope, or by varying the pressure). Others work by chemical or physical removal or sequestration of the impurity. For example, to purify ethanol beyond 95%, a drying agent (or desiccant, such as potassium carbonate) can be added to convert soluble water to insoluble water of crystallization. Molecular sieves are often used for this purpose as well.
Immiscible liquids, such as water and toluene, easily form azeotropes. Commonly, these azeotropes are referred to as a low boiling azeotrope because the boiling point of the azeotrope is lower than the boiling point of either of the pure components. The temperature and composition of the azeotrope are easily predicted from the vapor pressure of the pure components, without the use of Raoult's law. The azeotrope is easily broken in a distillation setup using a liquid-liquid separator (a decanter) to separate the two liquid layers that condense on top. Only one of the two liquid layers is refluxed to the distillation configuration.
High-boiling azeotropes also exist, such as a mixture of 20 weight percent hydrochloric acid in water. As the name implies, the boiling point of the azeotrope is higher than the boiling point of either of the pure components.
To break azeotropic distillations and cross distillation limits, as in the DeRosier Problem, it is necessary to increase the composition of the light key in the distillate.
Rupture of an azeotrope by unidirectional pressure manipulation
The boiling points of the components in an azeotrope overlap to form a band. By exposing an azeotrope to a vacuum or positive pressure, it is possible to shift the boiling point of one component away from the other by exploiting the different vapor pressure curves of each; the curves may overlap at the azeotrope, but it is unlikely that they will remain identical along the pressure axis on either side of the azeotrope. When the bias is large enough, the two boiling points no longer overlap and the azeotrope band disappears.
This method can eliminate the need to add other chemicals to a distillation, but it has two potential drawbacks.
Under negative pressure, power is needed for a vacuum source and the low boiling points of distillates require the condenser to be cooled to prevent distillate vapors from being lost to the vacuum source. Increased cooling demands will often require additional power and possibly new equipment or refrigerant change.
Alternatively, if positive pressures are required, standard glassware cannot be used, energy must be used for pressurization, and there is a greater chance of distillation side reactions such as decomposition due to the higher temperatures. high temperatures required to effect boiling.
A one-way distillation will depend on a pressure change in one direction, either positive or negative.
Pressure distillation
Pressure swing distillation is essentially the same as the one-way distillation used to break azeotropes, but both positive and negative pressures can be used here.
This improves the selectivity of the distillation and allows a chemist to optimize the distillation by avoiding energy-wasting extremes of pressure and temperature. This is particularly important in commercial applications.
An example of the application of pressure swing distillation is during the industrial purification of ethyl acetate after its catalytic synthesis from ethanol.
Industrial distillation
Large-scale industrial distillation applications include batch and continuous fractional distillation, vacuum, azeotropic, extractive, and steam distillation. The most commonly used industrial applications for continuous and steady-state fractional distillation are oil refineries, petrochemical and chemical plants, and natural gas processing plants.
To control and optimize said industrial distillation, a standardized laboratory method, ASTM D86, is established. This test method is extended to atmospheric distillation of petroleum products using a laboratory batch distillation unit to quantitatively determine the boiling range characteristics of petroleum products.
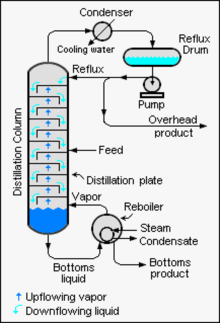
Industrial distillationis typically performed in large vertical cylindrical columns known as distillation towers distillation columns or distillation columns with varying diameters from about 65 centimeters to 16 meters and heights ranging from about 6. meters to 90 meters or more. When the process feed has a diverse composition, as in the distillation of crude oil, the liquid outlets at intervals up the column allow the extraction of different fractions or products that have different boiling points or ranges of concentrations. boiling. The "lightest" (those with the lowest boiling point) leave the top of the columns and the "heavier" (the ones with the highest boiling point) come out of the bottom of the column and are often called bottoms.
Industrial towers use reflux to achieve a more complete separation of products. Reflux refers to the portion of the liquid product condensed from the top of a distillation or fractionation tower that is returned to the top of the tower as shown in the schematic diagram of a typical large scale industrial distillation tower. Inside the tower, the descending reflux liquid provides cooling and condensation of the rising vapors, increasing the efficiency of the distillation tower. The more reflux that is provided for a given number of theoretical plates, the better the tower separates the low boiling materials from the higher boiling materials. Alternatively, the more reflux provided for a given desired separation, the fewer the number of theoretical plates required. Chemical engineers must choose what combination of reflux rate and number of plates is economically and physically feasible for the products purified in the distillation column.
These industrial fractionation towers are also used in cryogenic air separation, producing high purity liquid oxygen, liquid nitrogen and argon. The distillation of chlorosilanes also allows the production of high purity silicon for use as a semiconductor.
The design and operation of a distillation tower depend on the feed and desired products. Given a simple binary component feed, analytical methods such as the McCabe-Thiele method or the Fenske equation can be used. For a multi-component feed, simulation models are used for both design and operation.. In addition, the efficiencies of the vapor-liquid contact devices (referred to as "plates" or "trays") used in distillation towers are typically lower than those of a theoretical equilibrium stage. 100% efficient. Therefore, a distillation tower needs more trays than the number of theoretical vapor-liquid equilibrium stages. A variety of models have been postulated to estimate tray efficiencies.
In modern industrial applications, column packing material is used instead of trays when low pressure drops across the column are required. Other factors that favor packing are: vacuum systems, smaller diameter columns, corrosive systems, systems prone to foaming, systems requiring low liquid retention, and batch distillation. Conversely, factors favoring plate columns are: presence of solids in the feed, high rates of liquids, large column diameters, complex columns, columns with wide variation in feed composition, columns with a chemical reaction, absorption columns, columns limited by the tolerance of the foundation weight, low liquid rate, large turndown ratio and processes subject to process increases.
This packaging material can be packaged randomly dumped (1–3 "wide) as Raschig rings or structured sheet metal. Liquids tend to wet the packing surface, and vapors pass through this wet surface, where mass transfer takes place. Unlike conventional tray distillation in which each tray represents a separate vapor-liquid equilibrium point, the vapor-liquid equilibrium curve in a packed column is continuous. However, when modeling packed columns, it is useful to calculate several "theoretical stages" to denote the separation efficiency of the packed column relative to more traditional trays. Different shaped gaskets have different surface areas and void spaces between the gaskets. Both factors affect the performance of the packaging.
Another factor besides packing shape and surface area that affects random or structured packing performance is the distribution of liquid and vapor entering the packed bed. The theoretical number of stages required to make a given separation is calculated using a specific vapor to liquid ratio. If the liquid and vapor are not evenly distributed across the surface tower area when it enters the packed bed, the liquid to vapor ratio will not be correct in the packed bed and the required separation will not be achieved. The packaging will appear to be malfunctioning. The height equivalent to a theoretical plate (HETP) will be higher than expected. The problem is not the packing itself, but the poor distribution of the fluids that enter the filled bed. The maldistribution of the liquid is more frequent the problem than the vapor. The design of the liquid manifolds used to introduce feed and reflux into a packed bed is critical to the packing operating at maximum efficiency. Methods for evaluating the effectiveness of a liquid distributor in uniformly distributing incoming liquid into a packed bed can be found in references. Fractionation Research, Inc. (commonly known as FRI) has done considerable work on this topic.
Multi-effect distillation
The goal of multiple effect distillation is to increase the energy efficiency of the process, for use in desalination or, in some cases, a stage in the production of ultrapure water. The number of effects is inversely proportional to the kW·h/m³ of recovered water, and refers to the volume of recovered water per unit of energy compared to single-effect distillation. An effect is about 636 kW·h/m 3.
- Several-stage flash distillation can achieve more than 20 effects with thermal power input, as mentioned in the article.
- Steam compression evaporation (according to manufacturers) large-scale commercial units can achieve about 72 effects with power input.
There are many other types of multi-effect distillation processes, including one called simply multi-effect distillation (MED), which uses multiple chambers, with intervening heat exchangers.
Distillation in food processing
Distilled spirits
Carbohydrate-containing plant materials are left to ferment, producing a dilute solution of ethanol in the process. Spirits such as whiskey and rum are made by distilling these dilute solutions of ethanol. Components other than ethanol, including water, esters, and other alcohols, collect in the condensate, which accounts for the beverage's flavor. Some of these beverages are stored in barrels or other containers to acquire more flavor compounds and characteristic flavors.
Gallery
Sources Cited
- Forbes, R. J. (1970). A Short History of the Art of Distillation from the Beginnings up to the Death of Cellier Blumenthal. BRILL. ISBN 978-90-04-00617-1.
- Harwood, Laurence M.; Moody, Christopher J. (1989). Experimental organic chemistry: Principles and Practice (Illustrated edition). Oxford: Blackwell Scientific Publications. ISBN 978-0-632-02017-1.
Further reading
- Allchin, F. R. (1979). «India: The Ancient Home of Distillation?». Man 14 (1): 55-63. doi:10.2307/2801640.
- Needham, Joseph (1980). Science and Civilisation in China. Cambridge University Press. ISBN 0-521-08573-X.
- Geankoplis, Christie John (2003). Transport Processes and Separation Process Principles (4th edition). Prentice Hall. ISBN 978-0-13-101367-4.
Contenido relacionado
Robotics laws
Lipid bilayer
Annex: periodic table