Diamine
General structure of the diamines (primary). Primary amino groups (NH2) are marked in blue, A is a divalent organic radical (e.g. for- phenylene group). |
The diamine is an organic substance in whose molecule there are two -NH2 groups attached to one or two carbons of hydrocarbon radicals. The simplest and most well-known is ethylenediamine with the composition H2N - CH2 - CH2 - NH2, being a liquid with an odor similar to ammonia whose boiling point is 116 °C.
Diamines are used as monomers to prepare polyamides and polyureas. The term diamine mainly refers to the primary diamines, as they are the most reactive.
Regarding the quantities produced, 1,6-hexanediamine (a precursor of Nylon 6-6) is the most important, followed by ethylenediamine. The vicinal diamines (1,2 -diamines) are a structural motif in many biological compounds and are used as ligands in the chemistry of coordination compounds.
Aliphatic diamines
Linears
- 1 carbon: methandiamine (diaminomethane)
- 2 carbons: ethylenediamine (1.2-diaminoethane). Related derivatives include N-alkylated compounds, 1,1-dimetilendiamine, 1,2-dimethylenediamine, etambutol and TMEDA.
- 3 carbons: 1.3-diaminopropane (propano-1,3-diamine)
- 4 carbons: putrescina (butano-1,4-diamine)
- 5 carbons: perverine (pentano-1,5-diamine)
- 6 carbons: hexametilenediamine (hexano-1,6-diamine), trimetilhexametilenediamine
Branches
Derivatives of ethylenediamine are prominent:
- 1,2-diaminopropane, which is a chiral.
- dienilethyndiamine, which is C2.-simetric
- trans-1,2-diaminocyclohexane, which is C2-simetric.
Cyclic
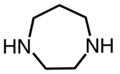
- 1,4-diazacicloheptano
Xylylenediamines
Xylylenediamines are classified as alkylamines since the amine is not directly attached to an aromatic ring.
o-xylenediamine or OXD
m-xylylenediamine or MXD
p-xylylenediamine or PXD
Aromatic diamines
Three phenylenediamines are known:
o-phenylenediamine or OPD
m-phenylenediamine or MPD
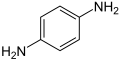
p-phenylenediamine or PPD. 2,5-diaminotoluene is related to PPD but contains a ring methyl group.
Several N-methylated derivatives of phenylenediamines are known:
- dimetil-4-fenilendiamine, a reactive.
- N,N'-di-2-butil-1,4-fenilendiamine, an antioxidant.
Examples with two aromatic rings include biphenyl and naphthalene derivatives:
- 4,4'-diaminobiphenyl
- 1.8-diaminonaphthalene
Contenido relacionado
Crystallography
Ligand
Princess of Asturias Award for Scientific and Technical Research
Polyoxometalate
Miller and Urey experiment