Dew point
The dew point or dew temperature is the highest temperature at which water vapor contained in the air begins to condense, producing dew, mist, any type of cloud or, in case the temperature is low enough, frost.
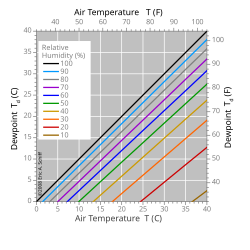
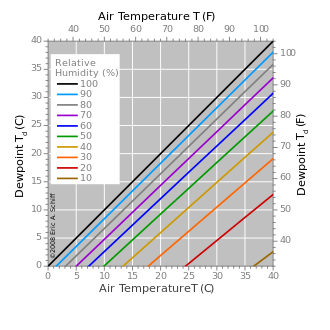
For a given mass of air, which contains a given amount of water vapor (absolute humidity), it is said that the relative humidity is the proportion of vapor contained in relation to that necessary to reach the saturation point, that is, to the dew point, and is expressed as a percentage. Thus, when the air becomes saturated (relative humidity equal to 100%), the dew point is reached. Saturation is produced by an increase in absolute humidity with the same temperature, or by a decrease in temperature with the same absolute humidity.
For there to be dew, the temperature must be at 0 °C or more, for example (3 °C or 5 °C)
Humidity
If all other factors that influence humidity remain constant, at ground level the relative humidity increases as the temperature drops; this is because less steam is needed to saturate the air. Under normal conditions, the dew point temperature will not be higher than the air temperature, since the relative humidity normally does not exceed 100%.
In technical terms, the dew point is the temperature at which water vapor in an air sample at constant barometric pressure condenses into liquid water at the same rate at which it evaporates. dew point, the rate of condensation will be greater than the rate of evaporation, forming more liquid water. Condensed water is called dew when it forms on a solid surface, or frost if it freezes. In air, condensed water is called a fog or a cloud, depending on the altitude at which it forms. If the temperature is below the dew point and no dew or mist is formed, the vapor is called supersaturated. This can occur if there are not enough particles in the air to act as condensation nuclei.
A high relative humidity implies that the dew point is close to the actual air temperature. A relative humidity of 100% indicates that the dew point is equal to the current temperature and that the air is maximally saturated with water. When the moisture content remains constant and the temperature increases, the relative humidity decreases, but the dew point remains constant.
General aviation pilots use dew point data to calculate the probability of carb icing and fog, and to estimate the height of the cumuliform cloud base.
Increasing barometric pressure increases the dew point. This means that if the pressure increases, the mass of water vapor per unit volume of air must decrease to maintain the same dew point. For example, consider New York City (elevation 10.1 m) and Denver (elevation 1,609.3 m). Since Denver is higher than New York, it will tend to have lower barometric pressure. This means that if the dew point and temperature in both cities are the same, the amount of water vapor in the air will be higher in Denver.
Calculation of dew point
An approximation used to calculate the dew point, Tdp, when the air temperature ("dry bulb") is known, T (in degrees Celsius) and relative humidity (in percent), RH, is the Magnus formula:
for more precision, Ps(T) (and thus γ( T, RH)) can be improved, using part of the Bögel modification, also called the Arden Buck equation, which introduces a fourth constant d:
- a = 6.1121 mbar, b = 18,678, c = 257.14 °C, d = 234.5 °C.
There are several sets of constants in use. Those used by NOAA are taken from a 1980 paper by David Bolton in the Monthly Weather Review:
- a = 6.112 mbar, b = 17.67, c = 243.5 °C.
These values provide a maximum error of 0.1%, for T in the range −30 °C ≤ T ≤ 35 °C and relative humidity 1% < HR < 100%. There is also a set of Sonntag1990 constants,
- a = 6.112 mbar, b = 17.62, c = 243.12 °C; for −45 °C ≤ T ≤ 60 °C (error ±0.35 °C).
Another common set of values comes from the Psychrometry and Psychrometric Charts published in 1974, presented in Paroscientific,
- a = 6.105 mbar, b = 17.27, c = 237.7 °C; for 0 °C ≤ T ≤ 60 °C (error ±0.4 °C).
Also in the Journal of Applied Meteorology and Climatology, Arden Buck presents several different sets of estimates, with different maximum errors for different temperature ranges. Two particular sets provide a range of −40 °C to +50 °C between the two, with an even smaller maximum error within the indicated range than all previous sets:
- a = 6.1121 mbar, b = 17.368, c = 238.88 °C; for 0 °C ≤ T ≤ 50 °C (error ≤ 0.05%).
- a = 6.1121 mbar, b = 17.966, c = 247.15 °C; for −40 °C ≤ T ≤ 0 °C (error ≤ 0.06%).
Simple approximation
There is also a very simple approximation that allows conversion between dew point, temperature and relative humidity. This approximation is accurate to about ±1 °C as long as the relative humidity is greater than 50%:
This can be expressed simply as:
For each difference of 1 °C in the temperatures of the dew point and dry bulb, relative humidity decreases 5%, starting with an RH = 100% when the dew temperature is equal to the humid bulb temperature.
The derivation of this approximation, a discussion of its accuracy, comparisons with other approximations, and more information on the history and applications of the dew point, can be found in an article published in the Bulletin of the American Meteorological Society.
Example
Making an application example:
| Symbol | Name | Unit |
|---|---|---|
| Pr{displaystyle Pr} | Dew point | |
| T{displaystyle T} | Temperature | °C |
| H{displaystyle H} | Relative humidity | % |
However the widely used formula is:
| Symbol | Name | Unit |
|---|---|---|
| Pr{displaystyle Pr} | Dew point | |
| T{displaystyle T} | Temperature | °C |
| H{displaystyle H} | Relative humidity | % |
This last formula, although widely used, does not always produce the correct result.
The dew point temperature also depends on the pressure of the air mass, a fact that is not taken into account in the above formulas.
Dew point in compressed air
In the air there are multiple gases, mainly oxygen, nitrogen and water vapor. The latter, unlike the other two, is not stable. Dalton's law of partial pressures allows us to analyze their behavior: "In a mixture of gases, the total pressure of the mixture is the sum of the partial pressures of the gases that compose it."
- Ptotal=p1+p2+ +pn{displaystyle P_{text{total}}=p_{1}+p_{2}+cdots +p_{n}}}
The maximum amount of water vapor that air can hold is also determined by temperature. The dew point is the temperature at which water begins to condense. This water condensation is a major problem in compressed air installations.
To prevent water condensation, there are equipments that dry the air, reducing the levels of water vapor so that they do not harm the installation or the process. Dew point temperature is used to measure the degree of dryness of compressed air.
Important concepts:
- Atmospheric dew point: the temperature at which the water vapor begins to condense in nature (at the atmospheric pressure).
- Pressure dew point: the temperature at which the water vapor begins to condense with a pressure above the atmospheric (condensation temperature that affects a compressed air installation).
Relationship with human comfort
When the air temperature is high, the human body uses the evaporation of perspiration to cool itself, and the cooling effect is directly related to how quickly perspiration evaporates. The rate of evaporation of perspiration depends on how much moisture is in the air and how much moisture the air can hold. If the air is already saturated with moisture (humid), the perspiration will not evaporate. The body's thermoregulation will produce perspiration in an effort to keep the body at its normal temperature, even when the rate of sweat production exceeds the rate of evaporation, so one can be covered in sweat on humid days even without generating additional body heat (such as exercising).
As the air around the body is warmed by body heat, it will rise and be replaced by other air. If the air is blown away from the body by a natural breeze or by a fan, the sweat will evaporate more quickly, making the perspiration more effective in cooling the body. The more unevaporated perspiration, the greater the discomfort.
A wet bulb thermometer also uses evaporative cooling, so it provides a good measure to use in assessing comfort level.
There is also discomfort when the dew point is very low (below about 23.0°F). The drier air can make the skin more easily chapped and irritated. It will also dry out the airways. The US Occupational Safety and Health Administration recommends that indoor air be maintained at 20-24.5 degrees Celsius (68-76 °F) with a relative humidity of 20-60%, equivalent to one point of dew from about 4.0 to 16.5°C (according to simple rule of thumb calculation below).
Lower dew points, below 10 degrees Celsius (50.0 °F), correlate with lower ambient temperatures and require less cooling for the body. A lower dew point can be accompanied by a high temperature only when the relative humidity is extremely low, allowing for relatively effective cooling.
People living in tropical and subtropical climates get somewhat acclimated to higher dew points. Thus, a resident of Singapore or Miami, for example, might have a higher discomfort threshold than a resident of a temperate climate like London or Chicago. People used to temperate climates often start to feel uncomfortable when the dew point is above 15°C, while others may find dew points as low as 18°C comfortable. Most people in temperate zones will find dew points above 21°C oppressive and tropical, while people in hot and humid zones may not find it uncomfortable. Thermal comfort depends not only on physical environmental factors, but also on psychological factors.
Links
- Calculation of the dew point.
- Online calculation of Dew Point.
- Dew point in compressed air.
Contenido relacionado
Fibered
Gas hydrate
Cardano

![{displaystyle {begin{aligned}gamma (T,mathrm {RH})&=ln left({frac {mathrm {RH} }{100}}right)+{frac {bT}{c+T}};\[8pt]T_{mathrm {dp} }&={frac {cgamma (T,mathrm {RH})}{b-gamma (T,mathrm {RH})}};end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e172e1a233ea113da9f7a276cdbc1df2a8b5a1f8)
![{displaystyle {begin{aligned}P_{mathrm {s} }(T)&={frac {100}{mathrm {RH} }}P_{mathrm {a} }(T)=ae^{frac {bT}{c+T}};\[8pt]P_{mathrm {a} }(T)&={frac {mathrm {RH} }{100}}P_{mathrm {s} }(T)=ae^{gamma (T,mathrm {RH})}\&approx P_{mathrm {s} }(T_{mathrm {w} })-BP_{mathrm {mbar} }0.00066left(1+0.00115T_{mathrm {w} }right)left(T-T_{mathrm {w} }right);\[8pt]T_{mathrm {dp} }&={frac {cln {frac {P_{mathrm {a} }(T)}{a}}}{b-ln {frac {P_{mathrm {a} }(T)}{a}}}};end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bd71c575f79892b285e213164feba70c8815aa08)
![{displaystyle {begin{aligned}P_{mathrm {s,m} }(T)&=ae^{left(b-{frac {T}{d}}right)left({frac {T}{c+T}}right)};\[8pt]gamma _{mathrm {m} }(T,mathrm {RH})&=ln left({frac {mathrm {RH} }{100}}e^{left(b-{frac {T}{d}}right)left({frac {T}{c+T}}right)}right);\[8pt]T_{dp}&={frac {cln {frac {P_{mathrm {a} }(T)}{a}}}{b-ln {frac {P_{mathrm {a} }(T)}{a}}}}={frac {cln left({frac {mathrm {RH} }{100}}{frac {P_{mathrm {s,m} }(T)}{a}}right)}{b-ln left({frac {mathrm {RH} }{100}}{frac {P_{mathrm {s,m} }(T)}{a}}right)}}={frac {cgamma _{m}(T,mathrm {RH})}{b-gamma _{m}(T,mathrm {RH})}};end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5fb24ff15b649d4d29abf5f724e8e66ad7cd7b66)
![{displaystyle {begin{aligned}T_{mathrm {dp} }&approx T-{frac {100-mathrm {RH} }{5}};\[5pt]mathrm {RH} &approx 100-5(T-T_{mathrm {dp} });end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/aad60925263f7cd19bed98cf8f153d1e1c67de95)
![{displaystyle Pr= {sqrt[{8}]{frac {H}{100}}}cdot (110+T) - 110}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1d559ecc0717635173506cf61d242a7adcfdf8c2)



![{displaystyle Pr= {sqrt[{8}]{frac {H}{100}}}cdot (112+0.9cdot T)+(0.1cdot T)-112}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7dd32e64e570517aff2fafdcf7d8201809a0f1d0)
