Cystic fibrosis
Cystic fibrosis (abbreviation CF) is an autosomal recessive genetic disease that mainly affects the lungs, and to a lesser extent the pancreas, liver, and intestines. causing the accumulation of thick and sticky mucus in these areas. It is one of the most common types of chronic lung disease in children and young adults, and it is a life-threatening disorder; patients often die from lung infections due to Pseudomonas or Staphylococcus.
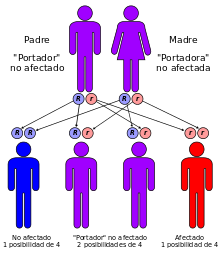
It is produced by a mutation in the gene that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein. This protein is involved in the passage of the chlorine ion through cell membranes and its deficiency alters the production of sweat, gastric juices and mucus. The disease develops when neither of the two alleles is functional. More than 1,500 mutations have been described for this disease, most of them are small deletions or point mutations; Less than 1% are due to promoter mutations or chromosomal rearrangements.
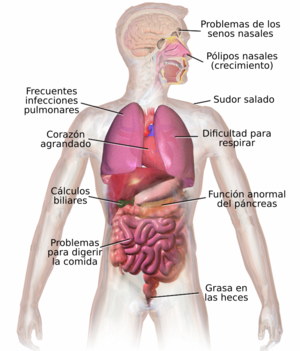
CF affects multiple organs and systems, causing abnormal and thick secretions from the exocrine glands. The main cause of morbidity and mortality is lung involvement, which is the cause of 95% of deaths, especially due to repeated infections caused by bronchial obstruction due to very thick mucus secretion. Other organs affected are the pancreas and sometimes the testicle.
It is one of the most frequent genetic diseases in the Caucasian race, with an incidence in this population of approximately 1/5000 live births. It is estimated that one in 25 people of European descent carry a non-functional allele.
The name cystic fibrosis refers to the characteristic processes of scarring (fibrosis) and cyst formation within the pancreas, first recognized in the 1930s. It is also known as mucoviscidosis (from the Latin muccus, 'mucus', and viscōsus, 'sticky').
Patients have a high concentration of salt (NaCl) in their sweat, which makes it possible to reach a diagnosis by analyzing it, performing the sweat test. Also through prenatal genetic testing, natal through gibson and cooke.
There is no curative treatment, however, there are treatments that improve symptoms and extend life expectancy. In severe cases, worsening disease may dictate the need for a lung transplant. The mean worldwide survival of these patients is estimated at 35 years, reaching higher values in countries with advanced healthcare systems; for example in Canada the average length of life was 48 years in 2010.
Symptoms and signs
The symptomatology of cystic fibrosis varies depending on the age of the individual, the degree to which specific organs are affected, the therapy previously instituted, and the types of associated infections. This disease compromises the organism in its entirety and shows its impact on growth, respiratory function, digestion. The neonatal period is characterized by poor weight gain and intestinal obstruction caused by dense and bulky stools. Other symptoms appear later, during childhood and early adulthood. These include growth retardation, advent of lung disease, and increasing difficulties from malabsorption of vitamins and nutrients in the gastrointestinal tract.
Most children are diagnosed with cystic fibrosis before the first year of life, when the sticky mucus that affects the lungs and pancreas begins to show its impact. In the respiratory tract, these secretions serve as a breeding ground for various bacteria responsible for chronic infections, with progressive and permanent deterioration of the lung parenchyma. As the respiratory condition worsens, patients develop pulmonary hypertension. On the other hand, in the pancreas, the mucus obstructs the transit of enzymes synthesized by the gland and prevents them from reaching the intestine to digest and absorb food.
Lung and Sinus Disease
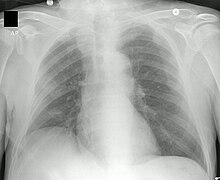
Lung disease results from blockage of the smaller airways with the thick mucus characteristic of cystic fibrosis. Inflammation and infection cause lung damage and structural changes that lead to a variety of symptoms. In the early stages, incessant coughing, copious production of phlegm, and a decrease in aerobic capacity are commonly present. Many of these symptoms occur when certain bacteria (mainly Pseudomonas aeruginosa) that normally live in thick mucus grow out of control and cause pneumonia. In advanced stages of CF, changes in the lung architecture lead to chronic respiratory difficulties.
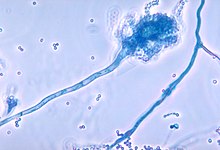
Other symptoms include coughing up blood or bloody sputum, chronic dilation of the bronchi or bronchioles (bronchiectasis), elevated blood pressure in the lungs, heart failure, feeling of not getting enough oxygen or breathlessness, respiratory failure, and atelectasis; ventilatory support may be required. In addition to the more common bacterial infections, people with CF more easily develop other types of respiratory illnesses. Among these is allergic bronchopulmonary aspergillosis, characterized by a hypersensitivity response to an ordinary fungus (mold) of the genus Aspergillus (Aspergillus fumigatus ), which exacerbates respiratory problems. Another example is infection with the Mycobacterium avium complex (MAC), a group of actinobacteria related to Mycobacterium tuberculosis, which can cause further damage to the lungs, and which does not respond to therapy with conventional antibiotics.
Mucus in the sinuses is equally thick and sticky, and can also cause occlusion of the openings where the sinuses normally drain, causing secretions to accumulate that act as breeding grounds for the aforementioned pathogens. In these cases, facial pain, fever, profuse nasal discharge, and headaches may occur. In people with CF, an overgrowth of nasal tissue (polyps) is often seen as a result of inflammation from chronic sinus infection. These polyps can aggravate upper airway obstruction and intensify breathing difficulties.
Gastrointestinal, liver and pancreatic disease
Prior to the spread of prenatal and neonatal tests for Cystic Fibrosis, it was common for the disease to be detected when it was found that the newborn could not expel its first feces (meconium). Meconium can completely block the intestines and cause serious disorders. This condition, called meconium ileus, occurs in 10% of newborns with CF. Genetic variants in genes related to ion transport in the small intestine have recently been identified that predispose to the development of meconium ileus. The association of CF with protrusion of the internal rectal membranes (rectal prolapse) is also frequent, due to increased fecal volume, malnutrition, and increased intra-abdominal pressure due to chronic cough.
The glutinous mucus observed in the lungs has its correlate in the thick secretions of the Pancreas, the organ responsible for providing digestive juices that facilitate the chemical decomposition of food. These secretions prevent the movement of pancreatic enzymes into the intestine and cause irreversible damage to the pancreas, often accompanied by painful inflammation (pancreatitis). Digestive enzyme deficiency results in a failure to absorb nutrients, with subsequent excretion. of these in the feces: this disorder is known as malabsorption. Malabsorption leads to malnutrition and delayed growth and development, both due to low caloric bioavailability. People with CF have, in particular, problems absorbing vitamins A, D, E, and K. In addition to pancreatic involvement, they often experience chronic heartburn, xerostomia, intestinal obstruction from intussusception, and constipation. Older patients also develop distal intestinal obstruction syndrome caused by glutinous feces.
These secretions can also cause liver problems. The bile, produced by this viscera to facilitate digestion, could block the bile ducts, damaging adjacent tissues. Over time, this situation leads to cirrhosis. In this case, first-order functions are compromised, such as those involved in the neutralization of toxins, and in the synthesis of important proteins (for example, coagulation factors, responsible for blood coagulation).
Endocrine disease and growth
The pancreas contains the islets of Langerhans, which are responsible for producing insulin, a hormone that helps regulate blood glucose levels. Damage to the pancreas can cause islet cell loss and lead to diabetes. Furthermore, vitamin D supplemented by food is involved in the regulation of calcium and phosphorus. The low availability of this, due to malabsorption, leads to osteoporosis, increasing the risk of suffering fractures. Additionally, people with CF often present, in their hands and feet, a malformation called clubbing fingers, which is due to the effects of this chronic disease and hypoxia in his bones.
Retarded growth is a hallmark of this disease. Children with CF generally fail to gain weight and height at rates comparable to their peers; often, they only receive a proper diagnosis after the causes of this phenomenon are investigated. Determinants of growth retardation are multifactorial and include chronic lung infection, nutrient malabsorption in the gastrointestinal tract, and increased metabolic demand associated with chronic condition.
Cystic fibrosis can be diagnosed by newborn screening, sweat electrolyte testing, or genetic testing. As of 2006, in the United States, 10% of cases are detected shortly after birth (desirably no later than the fifth day of life) as part of neonatal screening programs, which identify elevated levels of the trypsin enzyme by means of TIR (immunoreactive trypsinogen test). If the test is positive (levels less than or equal to 60 ng/mL —nanograms per milliliter—), it should be repeated on day 24-28 of life to verify that the levels continue to be greater than or equal to 50 ng/mL (positive screening), otherwise the screening will ultimately be negative. However, in most countries these tests are not routinely performed. For this reason, it is common for those affected to receive a proper diagnosis only once symptoms force an evaluation for this disease. The most commonly used diagnostic test is the sweat test, described by Lewis E. Gibson and Robert E. Cooke in 1959, using quantitative electrophoresis (iontophoresis) with a sweat-stimulating drug (pilocarpine). This substance, which has a positive charge, is applied to a positive electrode (+), in contact with the skin. Then, through the passage of electric current, the drug migrates through the integument towards another oppositely charged electrode (−), placed at a certain distance, until it crosses the epidermis, producing stimulation of the sweat glands and causing controlled sweating. Sweat samples are then collected on filter paper or in a capillary tube and analyzed for sodium and chloride concentrations. People with CF have higher levels of these ions in their sweat. Once the sweat test has been positive, a more detailed and precise diagnosis is made, by identifying mutations in the CFTR gene.
There are several tests to identify possible complications and control the evolution of the disease. X-ray and CT images make it easier to detect signs of injury or infection in the lungs. The sputum culture, examined under a microscope, provides information regarding which are the responsible bacteria, and allows to choose the most effective antibiotics. Pulmonary function tests measure lung capacities, lung volumes, and how quickly they can be moved (airflow). By means of such examinations, it is possible to determine if a treatment with antibiotics is appropriate or to evaluate the response to it. Blood tests can identify liver problems, vitamin deficiencies, and reveal the onset of diabetes. DEXA or DXA (dual energy X-ray absorptiometry) devices are used as a test to determine the presence of osteoporosis. Finally, the quantification of fecal elastase facilitates the detection of insufficiency of digestive enzymes.
Pathophysiology
The protein synthesized from the CFTR gene binds to the outer membrane of cells in the sweat glands, lung, pancreas, and other affected organs. The protein crosses this membrane and acts as an ion channel connecting the internal part of the cell (cytoplasm) with the extracellular fluid. This channel is mainly responsible for controlling the passage of chloride to (and from) the internal environment. When the CFTR protein does not function correctly, this movement is restricted, retaining chloride in the extracellular space. Because chloride has a negative electrical charge, positively charged ions will also not be able to cross the cytoplasmic membrane, due to the electrostatic attraction exerted by chloride ions. Sodium is the most common of the ions present outside the cell, and the combination of sodium and chloride gives rise to sodium chloride, which is lost in large amounts in the sweat of individuals with CF. This loss of salt constitutes the basic argument to explain the diagnostic utility of the sweat test.
The mechanism by which this cellular dysfunction produces the clinical manifestations described above is not exactly known. One of the theories that tries to explain it suggests that the failure of the CFTR protein to transport chloride determines the accumulation of abundant mucus in the lungs, creating a propitious environment (rich in nutrients) for bacteria, which thus manage to evade the system. immune. It is also postulated that this abnormality in the CFTR protein induces a paradoxical increase in sodium and chloride uptake, which stimulates water reabsorption, and results in the formation of thick, dehydrated mucus. Other theories focus on the phenomenon of chloride movement out of the cell, which also causes drying of mucus and pancreatic and biliary secretions. In general, these hypotheses coincide in attributing the greatest disorders to the obstruction of the thinnest ducts by the thick and glutinous secretions in the different affected organs. This situation conditions chronic infection and promotes structural remodeling of the lung, in addition to producing pancreatic damage (mediated by agglomerated digestive enzymes), and obstruction of the intestines by large fecal boluses.
The role of chronic infection in lung disease
The lungs of people with Cystic Fibrosis are colonized and infected by bacteria from an early age. Microorganisms that spread in these patients thrive on the abnormal mucus that accumulates in the narrower airways. Glutinous mucus stimulates the development of bacterial microenvironments (biofilms) that are difficult for immune cells and antibiotics to penetrate. For its part, the lungs respond to the ongoing damage inflicted by thick secretions and chronic infections by gradually remodeling the lower respiratory tract (bronchiectasis), making infection even more difficult to eradicate.
Over time, both the type of bacteria that affect these patients and the specific characteristics with which they present themselves change. In an early stage, certain ordinary bacteria such as Staphylococcus aureus and Haemophilus influenzae colonize and infect the lungs. Later, however, Pseudomonas aeruginosa (and sometimes the Burkholderia cepacia complex, made up of different Burkholderia species) prevailed. Once disseminated through the respiratory tract, these bacteria adapt to the environment and develop resistance to conventional antibiotics. Pseudomonas can acquire certain special characteristics, leading to the formation of large colonies—these strains are known as “mucoid” Pseudomonas, and are rare in people free of the disease—.
One of the ways the infection spreads is through transmission between individuals with CF. In the past, it was common for individuals to participate in summer camps and other recreational activities together. Hospitals housed CF patients in a common area, and standard equipment (for example, nebulizers) was not sterilized between successive uses. This led to the transmission of very dangerous bacterial strains between groups of patients. Currently, the routine in health care establishments consists of isolating these patients from each other; In addition, the personnel in charge of their care must wear gowns and gloves to limit the proliferation of virulent bacterial strains. Frequently, patients affected by particularly dangerous bacteria receive care on days and in different buildings than those assigned to those without those infections. In addition to bacterial infection, CF patients are predisposed to fungal colonization because of the ability of some fungi to colonize the lower respiratory tract and because of the frequent courses of antibiotics required to control the disease. most frequently cultivated are Aspergillus fumigatus and Candida albicans, this colonization translates into a high rate of inflammatory response against fungi. The role of fungi in CF has been defined, although they are considered non-pathogenic, except in cases of invasive aspergillosis and allergic bronchopulmonary aspergillosis.
Genetics
This is an autosomal recessive disease. In its most common form, a single amino acid mutation (missing a phenylalanine at position 508) leads to failure of cellular transport and cell membrane localization of the CFTR protein. More than 1800 mutations have been described, most of them being small deletions, although with different effects, such as changes in the reading frame, changes in amino acids, premature termination of the protein or alterations in splicing (splicing) of mRNA.
The CFTR gene is located on the long arm of chromosome 7, at position 7q31.2, occupying 180,000 base pairs: more precisely, from chromosome pair 116,907,252 to 117,095,950. It is a large gene, which is 250 kb (kilobases) and includes 27 exons. It was located and sequenced by genetic mapping.
This gene encodes the synthesis of a 1480 amino acid ion channel, a protein that transports chloride ions across epithelial cells, and controls the regulation of other transporters. In people with cystic fibrosis, this protein is absent or is found in significantly lower proportions than usual.
The penetrance of the disease varies according to the allele, and in turn, the expression of the allele depends on the environment and the genome of the affected person.
Molecular Biology
There are various mechanisms by which these mutations cause problems in the CFTR protein. In particular, the ΔF508 mutation generates a protein that does not fold normally and ends up being degraded by the cell. Several common mutations in the Ashkenazi population result in the synthesis of proteins that are too short, due to an early termination of their production. Other less frequent mutations cause proteins that do not use energy properly, do not allow chloride to cross the membrane properly, or are degraded at a faster than normal rate. The deficiency in the transport of chlorine means that the cells do not expel water to the outside and therefore the mucus is thicker. Certain mutations can also lead to a decrease in the production of copies of the CFTR protein.
Structurally, the CFTR gene belongs to the so-called ABC transporter superfamily (acronym for ATP Binding cassette). The tertiary structure of the protein encoded by this gene, it consists of two domains capable of hydrolyzing adenosine triphosphate, which allows the protein to use energy in the form of ATP. Likewise, another pair of domains, each one made up of six alpha helices, enables the protein to pass through the cell membrane. Activation is accomplished by a phosphorylation reaction at a regulatory binding site, primarily by protein kinase A (PKA, EC 2.7.11.11—formerly called cAPK or cyclic adenosine monophosphate-dependent protein kinase). The carboxyl terminus (C-) of the protein is attached to the cytoskeleton by interaction with PDZ protein domains.
Diagnosis
Traditional diagnosis (non-molecular methods)
There are a number of tests that are commonly performed to determine abnormalities of cystic fibrosis-related metabolites (especially chlorine). Among them are:
- Electrolytes test in the sweat: Pylocarpine is administered by iontoforesis to stimulate sweat production and the salt concentration is measured in this.
- Test the difference of nasal electric potential.
- Immunoreactive trypsinogen test: it is a test that is performed on the blood and that measures the concentration of a pancreatic enzyme.
Molecular diagnosis
The Molecular Diagnosis of the disease is complex, since in November 2010 we found 1824 mutations described and most are punctual or small deletions. These mutations are grouped based on the effect they have on the gene and on the disease phenotype. In addition to the variability of the mutations themselves, different populations have different frequencies for them, so studies and diagnostic tests must be managed considering this aspect. However, the most common in most populations is the 508F deletion.
It must be taken into account that the distribution of alleles varies greatly in each population, so the tests must be adapted to detect the most common variants in the population being studied.
Currently, as soon as they are born, children undergo a genetic diagnosis by sequencing the CFTR gene to find out if they have the disease, since it is a treatable disease. The sooner treatment begins, the better quality of life and greater longevity.
- Indirect molecular diagnosis: basically performed through ligation analysis. It is carried out by:
- RFLP: old method based on restriction currently in disuse.
- Microsatellite markers: is the method currently followed. Studies are only valid within the same family tree.
- SNP markers: they will be used in the near future, as they have the advantage that the results obtained are applicable to non-related people.
- Direct molecular diagnosis: we could sequester the CFTR gene, but what is at the order of the day is the detection of mutations, which is basically done from two strategies:
- Mutation tracking: mutation detection techniques, but no mutation identification. Some of the most used in this process are gel electrophoresis with denaturalization gradient, as well as some variants of PCR. An example of this is the detection of the ΔF508 mutation. This detection is done thanks to a restriction enzyme that cuts the healthy allele that does not present the detection mentioned, which allows the product of PCR after the amplification to be less in the healthy allele than in which the mutation presents, thus seeing the difference.
- Identification of mutations: techniques based on specific hybridization with mutated allele. For example, ASO:dot blot, OLA (oligonucleotide ligation), or the most traditional southern blot test.
- ASO: dot blot: is used for detection of previously known defined mutations. Read the DNA amplification to study. It is then fixed to a membrane (without treatment or previous electrophoresis). Then it is hybridized with oligonucleotides specific to the allele of about 20 base pairs that are marked for subsequent detection. It is a simple, cheap, fast and safe technique if studied (as in the case of cystic fibrosis) a single gene with few mutations. Although it is expensive if you have to rehibber many times. For those cases we have SO: dot blot inverse, in which the membrane has fixed the different alleles and these are hybridized with the genome amplified product marked.
- OLA: It is the most used method currently in the cystic fibrosis study, but when sequencing arrives it will probably be moved by it. Two steps are needed: a first step consists of a multiplex PCR of the genes to study. Then two ligation cycles with thermostable and oligonucleotides specific to alleles. These first steps are done all in the same reaction tube. A study of the fragments by capillary electrophoresis is then performed. The advantages of this process is its automation, although it is costly due to the need for an automatic sequencer.
Prenatal diagnosis
Couples who are pregnant or planning a pregnancy can be tested for CFTR gene mutations to determine the chances that their child will be born with cystic fibrosis. The test is usually performed on one or both parents and, if a high risk of CF is detected, it is also performed on the fetus. Since prenatal diagnosis does not enable superior or alternative forms of treatment, the main reason it is carried out is, in practice, to provide the possibility of abortion in case the fetus presents with the disease. Couples testing for cystic fibrosis is widely offered in countries such as the United States, and the American College of Obstetricians and Gynecologists (ACOG) recommends testing for couples who have a history of CF among their partners. immediate family members or close relatives, as well as those at high risk due to their ethnic affiliation.
Because the development of CF in the fetus requires each parent to pass on one copy of the mutant CFTR gene, and because of the high cost of prenatal testing, testing is usually done on only one parent initially. If one turns out to be a carrier of a CFTR gene mutation, then the other is tested to determine the risk of their child having the disease. CF can result from more than a thousand different mutations and, as of 2006, it is not possible to carry out laboratory studies for each of them. The test refers to analyzing the blood for the most common ones, such as ΔF508—most commercially available modalities detect no more than 32 different variants. If the fact that a family has a rare mutation is known, the latter can be specifically searched for. As a consequence of the fact that not all known mutations are detected by current tests, a negative result does not guarantee that the child will be free of the disease. On the other hand, since the probed mutations are necessarily those most common in the groups At higher risk, testing in lower-risk ethnicities is less successful, since the most widespread mutations in these groups are less frequent in the general population.
Couples at risk often have additional tests during or before pregnancy. In vitro fertilization with preimplantation genetic diagnosis offers the possibility of examining the embryo before its placement in the uterus. This test is performed three days after fertilization and seeks to determine the presence of abnormal CFTR genes. If, in an embryo, two mutant CTRF genes are identified, it will be excluded from the transfer, implanting another one that has at least one normal gene.
During the course of pregnancy, tests can be done on both the placenta (chorionic villus sampling) and the amniotic fluid surrounding the fetus (amniocentesis), with the help of ultrasound. However, chorionic villus sampling correlates with risk of stillbirth at a rate of 1 in 100 and amniocentesis at 1 in 200, so it is essential to determine the benefits properly to weigh the risks before proceeding. with the test. Alternatively, some couples choose to undergo assisted reproductive techniques with donor eggs (using in vitro fertilization) or donor sperm (donor artificial insemination).
Treatment
A fundamental aspect in the treatment of cystic fibrosis is the control and treatment of lung damage caused by thick mucus and infections, in order to improve the patient's quality of life. For the treatment of chronic and acute infections, antibiotics are administered intravenously, inhaled, and orally. Mechanical devices and drugs (in the form of inhalers) are also used to control secretions, thus decongesting and clearing the airways. Other aspects of therapy relate to the treatment of diabetes with insulin, of pancreatic disease with enzyme replacement. Additionally, the efficacy of different procedures, such as transplantation and gene therapy, is postulated to resolve some of the effects associated with this disease.
A healthy diet, high exercise and aggressive antibiotic treatments are increasing the life expectancy of patients.
Antibiotics to Treat Lung Disease
Antibiotics are prescribed whenever there is suspicion of pneumonia or ongoing deterioration in lung function. Usually, they are chosen based on the history of infections that previously affected the patient. Many of the common bacteria in cystic fibrosis are resistant to a large number of antibiotics and require weeks of intravenous treatment with vancomycin, tobramycin, meropenem, ciprofloxacin, and piperacillin.
Prolonged therapy often requires hospitalization and placement of an indwelling intravenous line, such as a percutaneously inserted central catheter (PICC). Likewise, the simultaneous indication of antibiotics administered by inhalation, such as tobramycin, colistin and gentamicin, is frequent for several months, in order to improve lung function by preventing bacterial proliferation. Some oral antibiotics such as ciprofloxacin or azithromycin are sometimes used to help prevent infection or to control it once it is ongoing. In some cases years pass between successive hospitalizations, while in others hospitalization is required every year for treatment.
In prolonged treatment, several of the most common antibiotics (such as tobramycin and vancomycin) can cause hearing loss due to ototoxicity or kidney problems. In order to prevent such side effects, it is usual to quantitatively measure the concentrations of these drugs in the blood and, if necessary, adjust the dosage.
Other Methods of Treating Lung Disease
There are various techniques that are implemented in order to fluidize the sputum and facilitate its expectoration. Physiotherapy is used in the hospital setting; a therapist practices a series of maneuvers using pressure and percussion (patting) exerted on the outside of the chest (thorax) several times a day. The mechanical devices that act under the same principle as those basic postural drainage techniques include the high-frequency oscillatory ventilator and intrapulmonary percussive ventilation devices, of which there are portable models, adaptable for home use. Aerobic exercise is highly beneficial for people with cystic fibrosis, as it not only promotes sputum decongestion, but also improves cardiovascular health and general condition.
Substances administered by inhalation that help thin secretions and facilitate their expulsion include dornase alfa and hypertonic saline. Dornase alfa is a recombinant human deoxyribonuclease (DNase or DNase), which breaks down DNA in the sputum, thus reducing the viscosity of the latter. N-acetylcysteine (a derivative of the amino acid cysteine) also works to thin sputum, but available research and experience have shown that the benefits are negligible. Finally, bronchodilators such as salbutamol and salmeterol (both β2-adrenergic agonist agents) or ipratropium bromide (a cholinergic receptor antagonist, quaternary derivative of atropine) are used to increase the smaller airways by relaxing the bronchial smooth muscle.
As the pulmonary condition worsens, mechanical respiratory support may be required. At night, some patients must wear special masks that work by pushing airflow into the lungs. Non-invasive ventilation using a nasal mask and positive pressure (VPAP, for the acronym for variable positive airway pressure) helps prevent significant drops in blood oxygen levels during sleep. It can also be used in the course of respiratory physiotherapy to promote the expulsion of sputum. However, in severe cases, it may be necessary to implement invasive forms of respiratory support with endotracheal intubation (i.e., placement of a tube or tube into the trachea).).
Treatment of Other Aspects of CF
Newborns with meconium ileus typically require surgery; this is generally not the case in adults with distal intestinal obstruction syndrome. Treatment of pancreatic insufficiency based on replacement of depleted digestive enzymes allows the intestines to properly absorb nutrients and vitamins that would otherwise be lost in the feces. Even so, most individuals with CF must receive additional doses of vitamins A, D, E, and K from supplements, and follow a high-calorie diet. The diabetes that often accompanies CF is treated with insulin injections. The development of osteoporosis can be prevented with vitamin D and calcium supplementation, and is often treated with bisphosphonates. insertion of a feeding tube (gastrostomy) to increase caloric intake from additional nutrition; growth hormone injections are also given for this purpose.
Sinus infections are often treated with a long course of antibiotics. The development of polyps, as well as other pathological structural changes inside the nasal passages, can restrict airflow and complicate the condition. For this reason, surgical practice is frequent in order to relieve the obstruction and limit the development of new infections. Intranasal corticosteroids, such as fluticasone, are also administered to reduce inflammation. On the other hand, female infertility can be combated using assisted reproductive techniques. Those that affect men also have treatment: for example, through intracytoplasmic sperm injection.
Transplantation, CFTR protein modulators, and gene therapy
Lung transplantation is generally considered appropriate in people with progressive decline in lung function and increasing exercise intolerance (fatigue or muscle exhaustion out of proportion to the exercise performed). Although a single lung transplant is viable in other diseases, in CF patients both must be replaced, since otherwise, the bacteria lodged in the remaining organ could infect the one that has been transplanted. In addition, a pancreas or liver transplant may be performed simultaneously for the purpose of alleviating liver disease or diabetes. Lung transplantation is considered when lung function is affected to such an extent that survival is threatened or assistance with mechanical devices is required.
Gene therapy represents a promising avenue in the fight against disease. This technique seeks to insert a normal copy of the CFTR gene into affected cells. Due to the inability of retroviruses to reach non-dividing cells, clinical trials have been performed to insert genes into adenoviruses. These viruses are currently being used in trials in which the normal CFTR gene is delivered, by an aerosol method, to the epithelial cells lining the lungs (in vivo gene therapy). Adenoviruses are expected to insert the normal gene, inducing relevant chloride channel function in these cells.
Some studies have suggested that only 5-10% of normal CFTR protein gene expression is required to prevent the pulmonary manifestations of CF. integrated into the DNA of the host cell. Therefore, they are eventually lost, causing transient gene expression and the need for reintroduction of the vector. Various approaches have been proposed and numerous clinical studies have been initiated, but as of 2006, multiple obstacles remain, which will need to be overcome for gene therapy to be successful.
The other approach for the treatment of cystic fibrosis is given by the use of enhancers or modulators of the CFTR protein, repairing the underlying defect in the creation of said protein. There are several enhancers or correctors in different phases of research among which stand out: Ivacaftor, Lumacaftor, Tezacaftor. The Ivacaftor enhancer, formerly called VX770 and under the trade name Kalydeco, was initially intended exclusively for patients older than 6 years, and as of 2015 it was cleared by the FDA for patients 2 to 5 years of age. It is authorized for the treatment of the G551D mutation, accounting for less than 3% of all cystic fibrosis patients. In 2014, the FDA authorized this drug for use in the G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, and G1349D mutations. modulator can improve the quality of life of patients, with weight gain and lung health, reducing the probability of infection. The improvement in FEV1 is on average 10.4%. The cost of this therapy exceeds US$300,000 per year of chronic treatment (2014). The combination of lumacaftor/ivacaftor (formerly called VX-809 and under the trade name Orkambi) from the same laboratory Vertex Pharmaceuticals has been approved by the FDA and by the European Union, for those with the ΔF508 mutation (the most common form). in its homozygous form (two identical copies). Talks are currently being held with the different countries of the European Union to negotiate reimbursement for the drug. The association of ivacaftor/tezacaftor (previously called VX-661 and under the trade name of Symkevi) for those who possess the ΔF508 mutation in its heterozygous form (a different copy in each allele) is under investigation.
For type I mutations, usually called nonsense mutations, where a stop codon causes the creation of the protein to be truncated at a premature stage and therefore not functional, drugs are being tested that have shown some efficacy by making the ribosomes ignore the premature termination codons and finish generating the CFTR protein, this ability of the drugs is called "read-through" in the literature. The drug Ataluren (formerly called PTC124) from PTC Therapeutics is in Stage III investigation at the FDA with ambiguous results for the majority of patients with these mutations, although promising in patients without chronic antibiotic treatment.
Other types of drugs seek to open alternate channels for chlorine in the cell, although they are in earlier stages of research.
Currently, animal models of the disease have been made through genetic editing and modification, with particular success in pigs and ferrets.
Epidemiology
Among people of European descent, cystic fibrosis is the most common of the life-threatening autosomal recessive diseases. In the United States, approximately 30,000 individuals have CF; most are diagnosed at six months of age. Canada has about 3,000 people with this condition. It is estimated that one in 25 people of European descent and one in 29 people of Ashkenazi descent are carriers of a cystic fibrosis mutation. Although less common in these groups, approximately one in 46 Hispanic Americans, one in 65 Africans, and one in 90 Asians carry at least one abnormal CFTR gene. Argentina and Uruguay represent an exception in the American context. Latin America, with an incidence of cases much higher than the average for the region and very close to that registered in the United States or Canada, and a prevalence of healthy carriers in the general population between 1 in 30 and 1 in 25.
Cystic fibrosis is diagnosed in both men and women. For reasons that are only partly known, life expectancy at birth turns out to be higher among affected men than among women. This indicator tends to vary mainly depending on the scope and quality of care provided by health systems. public. In 1959, the median survival in children with CF was 6 months. For those born in 2006 in the United States, this value would rise to 36.8 years, according to data compiled by the Cystic Fibrosis Foundation. The life expectancy rate has evolved in a similar way for a good part of West, except for less developed countries, where significantly lower figures are reported, and in which the majority of the affected population does not survive beyond ten years of age.
The Cystic Fibrosis Foundation also compiles information on the lifestyle of American adults with CF. In 2004, the foundation reported that 91% of this population had completed high school, and 54% had accessed some form of university education. The employment data revealed that 12.6% of these adults were unable to work (being left out of the economically active population), and 9.9% were unemployed. On the other hand, the marital information indicated that 59% were single and 36% were married or living with a partner. In 2004, 191 women with CF were pregnant in the United States.
Theories on the prevalence of CF
It is estimated that the ΔF508 mutation may be up to 52,000 years old. Numerous hypotheses have been formulated trying to explain why a lethal mutation such as this has persisted and spread through the human population. Some common autosomal recessive diseases such as sickle cell anemia have revealed the ability to protect their carriers from other conditions, a concept known as heterozygous advantage. With the discovery that the cholera toxin requires its hosts to be normal CFTR proteins in order to function properly, it has been postulated that carriers of mutant CFTR genes gained the benefit of resistance to cholera and other causes of diarrhea. However, subsequent studies have not confirmed this hypothesis.
The presence of normal CFTR proteins is a necessary condition for the entry of Salmonella typhi (serotype of Salmonella enterica, gram-negative proteobacteria of the genus Salmonella >) in cells, suggesting that carriers of mutant CFTR genes might be resistant to typhoid fever. However, no in vivo study has confirmed this hypothesis. In either case, the low incidence of cystic fibrosis outside of Europe, in places where both cholera and typhoid fever are endemic, has no immediate explanation.
History
Although the full clinical spectrum of CF was not recognized until the 1930s, certain aspects were identified much earlier. Carl von Rokitansky described a case of fetal death with meconium peritonitis, a complication of meconium ileus associated with cystic fibrosis. Meconium ileus was first described in 1905 by Karl Landsteiner.
In 1938, Dorothy Andersen published an article titled "Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study." pathological») in the American Journal of Diseases of Children. In this way, she was the first investigator to define this nosological entity (called, at that time, "cystic fibrosis of the pancreas"), and to correlate it with prominent pulmonary and intestinal disorders. She also postulated that it was a recessive disease and used pancreatic enzyme replacement as a treatment for affected children. In 1952, Paul di Sant' Agnese discovered abnormalities in sweat electrolytes. Based on that evidence, the sweat test was developed and refined over the course of the next decade.
In 1985, researchers from London, Toronto, and Salt Lake City mapped the CFTR gene to chromosome 7q. Four years later, in 1989, Francis Collins, Lap-Chee Tsui, and John R. Riordan discovered the first CF mutation, ΔF508, on that chromosome. Investigations subsequent to that finding identified more than a thousand different mutations that give rise to the disease. Lap-Chee Tsui led the team of scientists from the Hospital for Sick Children (a teaching hospital in partnership with the University of Toronto) that discovered the gene responsible for CF. It is the first genetic disorder to be elucidated strictly through the process of reverse genetics. Because CFTR gene mutations are generally small, classical or formal genetic techniques were not able to pinpoint the mutant gene. Using protein markers, genetic linkage studies were able to map the chromosome mutation. 7. Chromosome walking and jumping techniques were then used to identify and sequence the gene. This gene was one of the first genes to be located and sequenced by genetic mapping, and some of the participants in this project, such as Francis Collins, were involved later at the Human Genome Project
Identifying the specific mutation responsible for CF in a patient may be useful in predicting the course of the disease. For example, patients homozygous for the ΔF508 mutation present, in almost all cases, pancreatic insufficiency and generally have a relatively severe degree of respiratory involvement. However, there are exceptions that indicate the possibility that additional factors (perhaps genes at other loci) are involved in the expression of the disease. On the other hand, the cloning of the CF gene has opened up the possibility of gene therapy, as described in the relevant section.
Contenido relacionado
Gonad
Compsognathus longipes
Cuenca Alta del Manzanares Regional Park