Cysteine
Cysteine (abbreviated as Cys or C) is an α-amino acid with the chemical formula HS-CH2-CHNH2-COOH. It is a non-essential amino acid, which means that it can be synthesized by humans. The codons that code for cysteine are UGU and UGC (UraciloGuanineUracil and UraciloGuanineCytosine). Cysteine contains a thiol group (-SH) in its side chain, which makes this amino acid, as a whole, considered polar and hydrophilic. The thiol part of the chain usually participates in enzymatic reactions, acting as a nucleophile.
The thiol is susceptible to oxidation to give rise to disulfide bridges derived from cysteines, which have an important structural role in many proteins. Cysteine is also called cystine, but the latter is a dimer of two cysteines linked by a disulfide bond.
History
The first isolation of the protein was achieved by the Swedish chemist Count Morner in 1899. Earlier, Professor Eugen Baumann by reducing cystine was also successful.
Fonts
Food source that provides us with L-cysteine
Although it is classified as a non-essential amino acid, in some cases, cysteine may be essential for infants, the elderly, and people with certain metabolic diseases or suffering from malabsorption syndromes. Cysteine is normally synthesized by the human body under normal physiological conditions, provided there is sufficient methionine. Cysteine is potentially toxic and is catabolized in the digestive system and in the blood plasma. Cysteine travels safely through the digestive tract and plasma and is rapidly reduced to the two cysteine molecules that enter the cell.
Cysteine is found in most foods with a high protein content, such as:
- Raw meat: it is found in raw meat, since the cysine is thermoplastic or is degraded to heat.
- Eggs: they have to be from ranch chicken and not from those we commonly find in the supermarket.
- Milk sprouts: obviously it doesn't have to be the one we found in the supermarkets, it's a pasture cow.
- Maternal Milk: This is what will help us in the course of our life.
- Vegetables: Vegetables have to be for example brocoli or asparagus.
Like other amino acids, cysteine has an amphoteric character.
Synthesis of cysteine: Cysteine beta synthase catalyzes the upper reaction and cystathionine gamma-lyase catalyzes the lower reaction. In animals, biosynthesis begins with the amino acid serine. Sulfur is derived from methionine which is converted to homocysteine via the intermediate S-adenosylmethionine. Cystathionine beta-synthetase then combines homocysteine and serine to form the asymmetric thioether cystathionine. The enzyme cystathionine gamma-lyase converts cystathionine to cysteine and alpha-ketobutyrate. In plants and bacteria, cysteine biosynthesis also begins from serine, which is converted to O-acetylserine by the action of the serine acetyltransferase enzyme (EC 2.3.1.30). The enzyme O-acetylserine (thiol)-lyase ((OAS-TL; EC 2.5.1.47), using sulfur in the form of hydrogen sulfide, converts this ester to cysteine by displacement of acetate...
Biological functions
The thiol group of cysteine is nucleophilic and easily oxidizable. Reactivity increases when the thiol is ionized and cysteine residues in proteins have pH values close to neutral, so thiols are often found in reactive form in the cell. Due to its high reactivity, the thiol group of cysteine has numerous biological functions.
Antioxidant glutathione precursor
Due to the ability of thiols to undergo redox reactions, cysteine has antioxidant properties. These antioxidant properties of cysteine are mostly expressed in tripeptide glutathiones that occur both in humans and in other organisms. The systematic availability of oral glutathione (GSH) is negligible, for this reason it must be biosynthesized from the amino acids that constitute it, such as cysteine, glycine and glutamic acid. Glutamic acid and glycine are found abundantly in most Western diets, so cysteine availability may be the limiting substrate.
Disulfide bridges
Disulfide bonds play an important role in the assembly and stability of some proteins, usually proteins secreted into the extracellular medium. Since most cellular compartments are reduced media, disulfide bonds are generally unstable in the cytosol, except for a few exceptions that we see below.
Disulfide bonds in proteins are formed by the oxidation of thiol groups of cysteine residues. The other amino acids that also contain sulfur, such as methionine, cannot form disulfide bonds. Very aggressive oxidants convert cysteine into the corresponding sulfanic acid and sulfonic acid. Cysteine residues have a valuable role in cross-linked proteins, since it increases the rigidity of proteins and also confers proteolytic resistance. Within the cell, disulfide bonds between cysteine residues support the secondary structure of polypeptides. Insulin is an example of proteins with cross-linked cysteines, where two separate peptide chains are connected by a pair of disulfide bonds. Protein disulfide isomerases catalyze the disulfide bond formation itself; the cell transfers dehydroascorbic acid to the endoplasmic reticulum. In nature, cysteines are generally oxidized to cystines, their only function being nucleophilic.
Iron-sulfide group precursors
Cysteine is an important source of sulfur in human metabolism. Sulfur from iron-sulfide groups and from nitrogenases is extracted from cysteine and is converted to alanine during the process.
Metal ion binding
Aside from iron-sulfide-proteins, many other metal cofactors in enzymes are binding for the thiol substituent of cysteine residues. Examples of this are zinc in zinc fingers and alcohol dehydrogenase; copper in cuprous blue proteins, iron in cytochrome P450; and nickel in [NiFe]-hydrogenase. The thiol group also has great affinity for heavy metals, so cysteine-containing proteins such as metallothionein will bind metals such as mercury, lead, and cadmium strongly.
Post-translational modifications
Apart from its oxidation to cystine, cysteine is involved in numerous post-translational modifications. The nucleophilic thiol group allows cysteine to conjugate other groups, such as in prenylation, ubiquitin ligases transfer ubiquitin to their pendants, proteins, and to caspases involved in proteolysis in the apoptotic cycle. Inteins (protein introns) normally act as aids for the catalytic cysteine. These roles for cysteine are typically limited to the intracellular milieu, where the milieu is reduced and cysteine is not oxidized to cystine.
Other metabolites
The decarboxylation product of cysteine is cysteamine, a biogenic amine that is a fundamental component of coenzyme A. The product of cysteine transamination is mercaptopyruvate, which can be degraded to pyruvate or reduced to mercaptolactate by several possible routes, depending on the organism. Many microorganisms and plants fix cyanide anions by nucleophilic substitution with the sulfhydryl to give cyanoalanine as a product, which can be hydrolyzed to aspartate. The sulfur of cysteine can be methylated to obtain a methionine homologue called S-methylcysteine.
Enzymes:
- EC 1.8.1.6 Cystina Reductasa
- EC 1.1.1.27 L-lactate dehydrogenase
- EC 2.1.1.63 S-Methyltransferase
- EC 2.3.1.30 Seine O-acetyltransferase
- EC 2.5.1.47 Sintase cysteine (O-acetil-L-serina sulfhidrilasa)
- EC 2.6.1.3 Transamineous cysteine
- EC 2.8.1.2 3-Mercaptopiruvato azufre transferasa
- EC 4.2.1.65 3-Cianoalanine hydratesa
- EC 4.4.1.8 Cistationin β-liasa
- EC 4.4.1.9 L-3-cianoalanine syntase
- EC 4.4.1.15 D-cysteine desulfhydrase
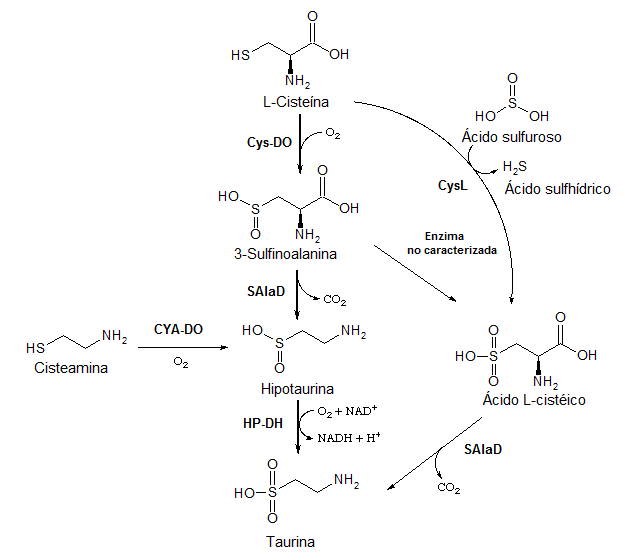
There is a group of metabolites that come from the oxidation of the sulfur atom of cysteine. Two examples of these metabolites are taurine and cysteic acid.
Enzymes:
- EC 1.8.1.3 Hipotaurina dehydrogenaseHP-DH)
- EC 1.13.11.19CYA-DO)
- EC 1.13.11.20Cys-DO)
- EC 4.1.1.29 Sulfoalanina descarboxilasa (SAlaD)
- EC 4.4.1.10 Liasa cysteine (Cysteine liasa)CysL)
Cysteine can be conjugated with an allyl group (of biosynthetic origin not yet reported), in order to give S-allylcysteine, which, when oxidized at the sulfur atom, generates alliin, the precursor of allyl disulfides and allyl sulfoxides that give onion and garlic aroma.
Applications
Cysteine, mainly as the L-enantiomer, is a precursor used in the food industry, the pharmaceutical industry, and the personal care industry. One of its major applications is the production of flavors. For example, the reaction of cysteine with sugars in the Maillard reaction produces flavors in meat. In the field of personal care products, cysteine is used as a perm predominantly in Asia, since cysteine is used to break disulfide bonds found in the keratin of hair. Cysteine is a very popular target in laboratories when investigating biomolecular structures. In 1994, in studies in five of the main tobacco companies, cysteine was observed to be one of the 599 additives in cigarettes. Like many tobacco additives, its purposeful use is unknown.
Sheep
Sheep need cysteine to make wool. For them it is an essential amino acid that must be ingested by eating grass. As a consequence, during periods of drought, the sheep stop producing wool. However, transgenic sheep have been developed that can produce their own cysteine.
Reducing the toxic effects of alcohol
Cysteine has been proposed as a preventative or antidote to some of the negative effects of alcohol, including liver damage and hangovers. Cysteine counteracts the damaging effects of acetaldehyde, which is the major product of alcohol metabolism and is responsible for most of the late effects of alcohol and long-term damage associated with alcohol use (but not referred to as immediate effects such as drunkenness). Cysteine is the next step in metabolism, which means converting acetylaldehyde to the relatively harmful acetic acid. In rodent studies, test animals received LD50 doses of aldehyde (the amount that normally kills half of all animals). Those given cysteine had an 80% survival rate, while those given added thiamine, they fully survived. There is still no clear evidence against its effectiveness in humans consuming alcohol at normal levels.
N-acetylcysteine (NAC)
N-acetyl-L-cysteine (NAC) is a derivative of cysteine in which the acetyl group is attached to the nitrogen atom. This compound can be considered as a dietary supplement, although this is not an ideal route, since it is catabolized in the intestine. NAC is usually used as a cough medicine, since it breaks the disulfide bridges in the mucosa, liquefying it, making it easier to expel. Also this action of breaking disulfide bridges makes NAC very useful in reducing abnormalities in the thickness of the mucosa in patients suffering from cystic fibrosis. NAC is also used as a specific antidote in cases of paracetamol overdose.
Contenido relacionado
Magnetochemistry
Scaphopoda
Molar conductivity