Copper
The copper (from the Latin cuprum, and this from the Greek kypros, Cyprus), whose symbol is Cu, it is the chemical element with atomic number 29. It is a copper-colored transition metal, that is, reddish-orange with a metallic luster that, together with silver, gold and roentgenium, forms part of the so-called copper family, and is characterized for being one of the best conductors of electricity (second only to silver). Thanks to its high electrical conductivity, ductility and malleability, it has become the most widely used material for manufacturing electrical cables and other electrical elements and electronic components.
Copper is part of a very large number of alloys that generally have better mechanical properties, although they have lower electrical conductivity. The most important are known by the name of bronzes and brasses. On the other hand, copper is a durable metal because it can be recycled an almost unlimited number of times without losing its mechanical properties.
It was one of the first metals to be used by humans in prehistoric times. Copper and its alloy with tin, bronze, became so important that historians have called two periods of Antiquity the Copper Age and the Bronze Age. Although its use lost relative importance with the development of the steel industry, copper and its alloys continued to be used to make objects as diverse as coins, bells, and cannons. From the XIX century, specifically from the invention of the electric generator in 1831 by Faraday, copper once again became a strategic metal, as it is the main raw material for cables and electrical installations.
Copper plays an important biological role in the process of photosynthesis in plants, although it is not part of the composition of chlorophyll. Copper contributes to the formation of red blood cells and to the maintenance of blood vessels, nerves, the immune system and bones and is therefore an essential trace element for human life.
Copper is found in a large amount of common foods in the diet such as oysters, shellfish, legumes, organ meats and nuts among others, as well as drinking water and therefore it is very rare for a copper deficiency to occur in the organism. The copper imbalance causes a liver disease in the body known as Wilson's disease.
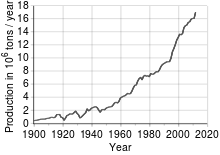
Copper is the third most widely used metal in the world, behind iron and aluminum. The world production of refined copper was estimated at 15.8 Mt in 2006, with a deficit of 10.7% compared to the projected world demand of 17.7 Mt. Porphyry copper constitutes the main source of copper extraction in the world.
Names and symbols
- Etymology
- The word "copper" comes from Latin cuprum (with the same meaning) and this in turn of the expression aes cyprium which literally means "of Cyprus" because of the great importance of the copper mines of the island of Cyprus in the Greco-Roman world.
- Abbreviations and abbreviations
- The current chemical symbol of copper is "Cu". Centuries ago, alchemists represented it with the symbol que that also represented the planet Venus, the Greek goddess Aphrodite and the female genus. The reason for this relationship may be that the Phoenician goddess Astarté, which is partly equivalent to Aphrodite, was very venerated in Cyprus, an island famous for its copper mines. The. symbol is similar to the Egyptian hieroglyph, which represented life or perhaps also sexual union. However, in the Greek mythology the divinity that presided over the manufacture of the copper coin was Esculano.
- Adjective
- The particular qualities of copper, specifically regarding its colour and lustre, have engendered the root of the qualifier Cobrizo. The same particularity of the material has been employed by colloquially naming some snakes from India, Australia and the United States as "copper head».
History
Copper in Antiquity
Copper is one of the few metals that can be found in nature in the form of native copper, that is, without combining with other elements. For this reason it was one of the first to be used by humans. The other native metals are gold, platinum, silver and iron from meteorites.
Native copper utensils have been found around 7000 BCE. C. in Çayönü Tepesí (in present-day Turkey). The copper from Çayönü Tepesí was annealed but the process was not yet perfected. Copper carbonates (malachite and azurite) were also used in ornamental patterns in the Near East at this time. In the Great Lakes region of North America, where native copper deposits were abundant, from 4000 B.C. C. the natives used to hit them to give them the shape of an arrowhead, although they never discovered the fusion.
The first crucibles to produce metallic copper from carbonates by carbon reduction date from the 5th millennium BC. C. It is the beginning of the so-called Copper Age, crucibles appearing throughout the area between the Balkans and Iran, including Egypt. Evidence of the exploitation of copper carbonate mines from very ancient times has been found both in Thrace (Ai Bunar) and in the Sinai Peninsula. Endogenously, not connected to Old World civilizations, in pre-Columbian America, around the 4th century B.C. C. the Moche culture developed the metallurgy of already refined copper from malachite and other copper carbonates.
Around 3500 B.C. C. copper production in Europe declined due to the depletion of carbonate deposits. Around this time there was an irruption from the east of some peoples, generically called kurgans, who brought a new technology: the use of arsenical copper. This technology, perhaps developed in the Near East or the Caucasus, made it possible to obtain copper through the oxidation of copper sulphide. To prevent the copper from oxidizing, arsenic was added to the ore. Arsenical copper (sometimes also called "arsenical bronze") was sharper than native copper and could also be obtained from the very abundant sulphide deposits. Coupled with the also new technology of the two-piece mold, which allowed the mass production of objects, the kurgans were equipped with tomahawks and spread rapidly.
Ötzi, the corpse found in the Alps and dated to around 3300 BC. C., carried a copper ax with 99.7% copper and 0.22% arsenic. The site of Los Millares (Almería, Spain), a metallurgical center near the mines of copper from the Sierra de Gádor.
It is not known how or where the idea of adding tin to copper, producing the first bronze, arose. It is believed to have been an unforeseen discovery, as tin is softer than copper, yet adding it to copper produced a harder material that held edges longer. The discovery of this new technology sparked the beginning from the Bronze Age, dated to around 3000 BCE. C. for the Near East, 2500 a. C. for Troy and the Danube and 2000 a. C for China. At the Bang Chian site in Thailand, bronze objects have been dated from before 2000 BC. C. For many centuries, bronze played a leading role and tin deposits became very important, often far from the large urban centers of that time.
The decline of bronze began around 1000 B.C. C., when a new technology emerged in the Near East that made it possible to produce metallic iron from iron ores. Iron weapons were replacing copper weapons throughout the space between Europe and the Middle East. In areas like China, the Bronze Age lasted several more centuries. There were also regions of the world where bronze was never used. For example, sub-Saharan Africa went directly from stone to iron.
However, the use of copper and bronze did not disappear during the Iron Age. Replaced in weapons, these metals began to be used essentially in construction and decorative objects such as statues. Brass, an alloy of copper and zinc, was invented around 600 B.C. C. Also around this time the first coins were made in the state of Lydia, in present-day Turkey. While the most valuable coins were minted in gold and silver, those for more everyday use were made of copper and bronze.
The search for copper and precious metals in the Mediterranean led the Carthaginians to exploit the large deposit of Río Tinto, in the current province of Huelva. After the Punic Wars, the Romans seized these mines and continued to exploit them until they exhausted all the copper oxide. Beneath him was a large vein of copper sulphide, which the Romans did not know how to take advantage of effectively. At the fall of the Roman Empire, the mine had been abandoned and was only reopened when the Andalusians invented a more efficient process to extract copper from sulphide.
Middle Ages and Modern Ages
The resistance to corrosion of copper, bronze and brass allowed these metals to have been used not only as decorative but also as functional from the Middle Ages to the present day. Between the 10th and 12th centuries, large deposits of silver and copper were found in Central Europe, mainly Rammelsberg and Joachimsthal. From them came a large part of the raw material to make the great bells, doors and statues of European Gothic cathedrals. In addition to the warlike use of copper for the manufacture of objects, such as axes, swords, helmets or breastplates; copper was also used in the Middle Ages in luminaries such as lamps or chandeliers; in braziers and in storage objects, such as chests or cases.
The earliest European wrought-iron cannons date from the 14th century, but around the XVI bronze established itself as the almost sole material for all artillery and held that dominance well into the XIX. In the Baroque, during the 17th and 18th centuries, copper and its alloys acquired great importance in the construction of monumental works, the production of watchmaking machinery and a wide variety of decorative and functional objects. The authoritarian monarchies of the Old Regime used copper in alloy with silver (called fleece) to carry out repeated monetary devaluations, reaching the point of issuing purely copper coins, characteristic of the difficulties of the Treasury of the Hispanic Monarchy of the century XVII (which used it in such quantity that it had to resort to importing it from Sweden).
Contemporary Age
During 1831 and 1832, Michael Faraday discovered that an electrical conductor moving perpendicular to a magnetic field generated a potential difference. Taking advantage of this, he built the first electric generator, the Faraday disk, using a copper disk that rotated between the ends of a horseshoe-shaped magnet, inducing an electric current. The subsequent development of electric generators and their use in history of electricity has given rise to the fact that copper has obtained outstanding importance in humanity, which has increased its demand remarkably.
For much of the 19th century, Britain was the world's largest producer of copper, but its growing importance copper motivated mining exploitation in other countries, with production in the United States and Chile standing out, as well as the opening of mines in Africa. Thus, in 1911 world copper production exceeded one million tons of fine copper.
The emergence of the processes that enabled the mass production of steel in the mid-19th century, such as the Thomas- Bessemer or the Martin-Siemens furnace gave rise to replacing the use of copper and its alloys in some specific applications where a more tenacious and resistant material was required. However, the technological development that followed the Industrial Revolution in all branches of human activity and the advances achieved in copper metallurgy have allowed the production of a wide variety of alloys. This has led to an increase in the fields of application of copper, which, added to the economic development of several countries, has led to a notable increase in world demand.
United States
Since the early 19th century there was copper production in the United States, first in Michigan and later in Arizona. These were small mines that exploited high-grade ore.
The development of the more efficient flotation process towards the end of the XIX century allowed large deposits of low law, primarily in Arizona, Montana, and Utah. In a few years, the United States became the world's largest producer of copper.
In 1916, American mines produced more than a million tons of copper for the first time, representing about three-quarters of world production. Mining production fell sharply after the 1929 crisis, not only due to reduced consumption but also because metal recycling skyrocketed. Demand picked up in the late 1930s, with US mines again surpassing one million tons in 1940. However, this figure already represented "only" half of world production and did not cover domestic demand, so in 1941 the country became a net importer of copper for the first time.
From the 1950s to the present, production in the United States has ranged from one to two million tons per year, which represents a diminishing fraction of the world total (27% in 1970, 17% in 1980, 8% in 2006). Meanwhile, consumption has continued to grow continuously and this has forced the importation of increasing amounts of metal, exceeding one million tons imported for the first time in 2001.
Chili
In 1810, the year of its first national junta, Chile produced about 19,000 tons of copper a year. Throughout the century, the number grew until the country became the world's leading producer and exporter. However, at the end of the XIX century, a period of decline began, due on the one hand to the exhaustion of high-grade deposits law and on the other to the fact that the exploitation of nitrate monopolized mining investments. By 1897 production had fallen to 21,000 tons, about the same as in 1810.
The situation changed at the beginning of the XX century, when large mining groups endowed with this country obtained technological advances that allowed the recovery of copper in deposits of low concentration, initiating the exploitation of the Chilean deposits.
The Chilean state received little benefit from copper mining throughout the first half of the XX century. The situation began to change in 1951 with the signing of the Washington Agreement, which allowed it to dispose of 20% of the production. In 1966, the National Congress of Chile imposed the creation of Mixed Mining Companies with foreign companies in which the State would have 51% ownership of the deposits. The process of Chileanization of copper culminated in July 1971, under the mandate of Salvador Allende, when Congress unanimously approved the nationalization of the Large Copper Mining.
[...] for demanding the national interest and in the exercise of the sovereign and inalienable right of the State to freely dispose of its natural wealth and resources, they are nationalized and therefore declared incorporated into the full and exclusive domain of the Nation foreign companies that constitute the great mining of copper.Transitional provision added in 1971 to article 10 of the Chilean Constitution.
In 1976, already under the military dictatorship of Pinochet, the State founded the National Copper Corporation of Chile (Codelco) to manage the large copper mines.
The Chuquicamata mine, in which evidence of copper extraction by pre-Columbian cultures has been found, began its construction for exploitation in 1910 and its industrial exploitation began on May 18, 1915. Chuquicamata is the It was the world's largest open pit mine and was for several years the world's highest producing copper mine. In 2002, the Chuquicamata and Radomiro Tomic divisions merged, creating the Codelco Norte mining complex, consisting of two mines open pit, Chuquicamata and Mina Sur. Although the Radomiro Tomic deposit was discovered in the 1950s, operations began in 1995, once the technical and economic feasibility studies were updated.
In 1995, the construction of the Minera Escondida mine began in the II Region of Antofagasta, and in 1998 extraction operations began. It is the mine with the highest production in the world. The Minera Escondida strike in 2006 paralyzed production for 25 days and altered world copper prices. In 2007, Minera Escondida's production reached 1,483,934 t. This production represents 9.5% of the world production and 26% of Chilean copper production, according to estimates for 2007.
In recent decades, Chile has established itself as the world's largest copper producer, going from 14% of world production in 1960 to 36% in 2006.
Isotopes
Two stable isotopes are found in nature: 63Cu and 65Cu. The lightest of them is the most abundant (69.17%). Up to now, 25 radioactive isotopes have been characterized, of which the most stable are 67Cu, 64Cu and 61Cu with periods half-life of 61.83 hours, 12.70 hours and 3.333 hours respectively. The other radioisotopes, with atomic masses from 54.966 amu (55Cu) to 78.955 amu (79Cu), have half-lives of less than 23.7 minutes and most do not reach 30 seconds. The isotopes 68Cu and 70Cu present metastable states with a half-life longer than the ground state.
Isotopes lighter than the stable 63Cu decay mainly by positive beta emission, giving rise to nickel isotopes, while those heavier than the stable 65Cu isotope they disintegrate by negative beta emission giving rise to zinc isotopes. The isotope 64Cu disintegrates generating 64Zn, by electronic capture and positive beta emission in 69% and by negative beta disintegration it generates 64Ni in the remaining 31%.
Copper properties and characteristics
Physical properties
Copper has several physical properties that favor its industrial use in multiple applications, being the third most consumed metal in the world, after iron and aluminum. It is reddish in color and has a metallic luster and, after silver, it is the element with the highest electrical and thermal conductivity. It is an abundant material in nature; it has an accessible price and is recycled indefinitely; forms alloys to improve mechanical performance and is resistant to corrosion and oxidation.
The electrical conductivity of pure copper was adopted by the International Electrotechnical Commission in 1913 as the standard reference for this quantity, establishing the International Annealed Copper Standard, or IACS. According to this definition, the conductivity of annealed copper measured at 20 °C is equal to 5.80 × 107 S/m. This conductivity value is assigned a 100% IACS index and the conductivity of the rest of the materials is expressed in percentage of IACS. Most metals have conductivity values less than 100% IACS but there are exceptions such as silver or the special very high conductivity coppers designated C-103 and C-110.
Mechanical properties
Both copper and its alloys have good machinability, that is, they are easy to machine. Copper has very good ductility and malleability, which allows it to produce very thin and fine sheets and wires. It is a soft metal, with a hardness index of 3 on the Mohs scale (50 on the Vickers scale) and its tensile strength is 210 MPa, with an elastic limit of 33.3 MPa. It supports manufacturing processes deformation such as rolling or forging, and welding processes and their alloys acquire different properties with heat treatments such as tempering and annealing. In general, its properties improve at low temperatures, which allows it to be used in cryogenic applications.
Chemical characteristics
In most of its compounds, copper has low oxidation states, the most common being +2, although there are also some with oxidation state +1.
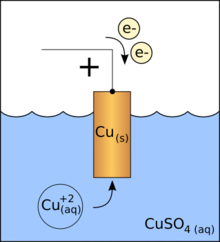
Exposed to air, the salmon-red color initially turns violet-red by the formation of cuprous oxide (Cu2O) to later blacken by the formation of cupric oxide (CuO). The blue coloration of Cu+2 is due to the formation of the ion [Cu (OH2)6]+2.
Exposed for a long time to humid air, it forms an adherent and impermeable layer of poisonous green basic carbonate (cupric carbonate). It can also form patinas of verdigris, a poisonous mixture of greenish or bluish copper acetates that It is formed when copper oxides react with acetic acid, which is responsible for the taste of vinegar and is produced in acetic fermentation processes. When using copper utensils for cooking food, precautions must be taken to avoid poisoning by verdigris which, despite its bad taste, can be masked with sauces and seasonings and be ingested.
Halogens easily attack copper, especially in the presence of moisture. When dry, chlorine and bromine have no effect and fluoride only attacks it at temperatures above 500 °C. Cuprous chloride and cupric chloride, combined with oxygen and in the presence of moisture, produce hydrochloric acid, causing stains of atacamite or paratacamite, pale green to blue-green in color, soft and powdery that does not settle on the surface and produces more copper chlorides, starting the cycle of erosion again.
Oxacid acids attack copper, which is why these acids are used as strippers (sulfuric acid) and brighteners (nitric acid). Sulfuric acid reacts with copper to form a sulphide, CuS (covelin) or Cu2S (chalcocite) which is black and water colored. Cupric sulphate (antlerite) salts with green to blue-green colors can also form. These salts are very common in the anodes of lead-acid batteries used in automobiles.
Citric acid dissolves copper oxide, so it is applied to clean copper surfaces, polishing the metal and forming copper citrate. If, after cleaning copper with citric acid, the same cloth is used again to clean lead surfaces, the lead will be coated in an outer layer of copper citrate and lead citrate with a reddish and black color.
Biological properties
In plants, copper plays an important role in the process of photosynthesis and is part of the composition of plastocyanin. About 70% of the copper in a plant is present in chlorophyll, mainly in chloroplasts. The first symptoms in plants due to copper deficiency appear in the form of narrow, twisted leaves, as well as whitish tips. Panicles and pods can appear empty due to a severe copper deficiency, causing serious economic losses in agricultural activity.
Copper contributes to the formation of red blood cells and the maintenance of blood vessels, nerves, the immune system and bones and is therefore essential for human life. Copper is found in some enzymes such as cytochrome c oxidase, lysyl oxidase and superoxide dismutase.
The imbalance of copper in the body when it is produced excessively causes a liver disease known as Wilson's disease, the origin of this disease is hereditary, and apart from the liver disorder that it causes, it also damages the nervous system. It is a rare disease.
Copper deficiency can occur in children with a diet low in calcium, especially if they have diarrhea or malnutrition. There are also diseases that decrease the absorption of copper, such as celiac disease, cystic fibrosis, or by having restrictive diets.
Copper is found in a large amount of common foods in the diet such as oysters, shellfish, legumes, organ meats and nuts among others, as well as drinking water and therefore it is very rare for a copper deficiency to occur in the organism.
Copper health precautions
Although copper is a trace element necessary for life, high levels of this element in the body can be harmful to health. Inhalation of high levels of copper may cause respiratory tract irritation. Ingestion of high levels of copper can cause nausea, vomiting, and diarrhea. Excess copper in the blood can damage the liver and kidneys, and even cause death. Taking 30 g of copper sulfate orally is potentially lethal in humans.
For work activities in which copper products are made and handled, it is necessary to use collective protection measures that protect workers. The tolerated limit value is 0.2 mg/m³ for smoke and 1 mg/m³ for dust and mist. Copper reacts with strong oxidants such as chlorates, bromates, and iodides, creating an explosion hazard. In addition, the use of personal protective equipment such as gloves, glasses and masks may be necessary. In addition, it may be advisable for workers to shower and change their clothes before returning home each day.
The World Health Organization (WHO) in its Guide to the quality of drinking water recommends a maximum level of 2 mg/l. The same value has been adopted in the European Union as limit value for copper in drinking water, while in the United States the Environmental Protection Agency has set a maximum of 1.3 mg/l. Water with copper concentrations greater than 1 mg/l can soil clothes when washed and have an unpleasant metallic taste. The United States Agency for Toxic Substances and Disease Registry recommends that, in order to decrease copper levels in drinking water through copper pipes, flush the water for at least least 15 seconds before drinking or using for the first time in the morning.
Mining activities can cause the contamination of rivers and subterranean waters with copper and other metals during their exploitation as well as once mining has been abandoned in the area. The turquoise color of the water and the rocks is due to the action that copper and other metals develop during mining.
Copper alloys and types
Physically, pure copper has a very low yield strength (33 MPa) and low hardness (3 on the Mohs scale or 50 on the Vickers scale). Instead, alloyed with other elements acquire much higher mechanical characteristics, although their conductivity decreases. There is a wide variety of copper alloys, the compositions of which depend on the technical characteristics obtained, which is why they are used in a multitude of objects with highly diverse technical applications. Copper is mainly alloyed with the following elements: Zn, Sn, Al, Ni, Be, Si, Cd, Cr and others to a lesser extent.
Depending on the purposes for which they are used in the industry, they are classified into alloys for forging and alloys for molding. To identify them, they have the following general nomenclatures according to the ISO 1190-1:1982 standard or its equivalent UNE 37102:1984. Both standards use the UNS system (from the English Unified Numbering System).
Brass (Cu-Zn)
Brass, also known as cuzin, is an alloy of copper, zinc (Zn) and, to a lesser extent, other metals. It is obtained by melting its components in a crucible or by melting and reducing sulfurous ores in a reverberatory or cupola furnace. In industrial brasses, the percentage of Zn is always kept below 50%. Its composition influences the mechanical characteristics, the fusibility and the ability to be formed by casting, forging and machining. When cold, the ingots obtained are plastically deformed to produce sheets, rods or cut into strips that can be stretched to make wires. Its density depends on its composition and is generally between 8.4 g/cm³ and 8.7 g/cm³.
The characteristics of brasses depend on the proportion of elements involved in the alloy in such a way that some types of brass are malleable only when cold, others exclusively when hot, and some are not malleable at any temperature. All types of brass become brittle when heated to a temperature close to the melting point.
Brass is harder than copper, but easy to machine, engrave, and cast. It is resistant to oxidation, to saline conditions and is malleable, so it can be rolled into thin sheets. Its malleability varies with temperature and with the presence, even in minimal amounts, of other metals in its composition.
A small contribution of lead in the brass composition improves machinability because it facilitates the fragmentation of chips in machining. Lead also has a lubricating effect due to its low melting point, which slows down the wear of the cutting tool.
Brass admits few heat treatments and only homogenization and recrystallization annealing is performed. Brass has a bright yellow color, resembling gold, a characteristic that is used in jewelry, especially costume jewelery, and in the galvanizing of decorative elements. Brass applications cover other very diverse fields, such as weapons, boilermaking, welding, wire manufacturing, condenser tubes, and electrical terminals. Since it is not attacked by salt water, it is also used in shipbuilding and in fishing and marine equipment.
Brass does not spark on mechanical impact, an atypical property for alloys. This characteristic makes brass an important material in the manufacture of containers for handling flammable compounds, metal cleaning brushes and lightning rods.
Bronze (Cu-Sn)
The alloys in whose composition copper and tin (Sn) predominate are known by the name of bronze and have been known since ancient times. There are many types of bronzes that also contain other elements such as aluminum, beryllium, chrome or silicon. The percentage of tin in these alloys is between 2 and 22%. They are yellowish in color, and bronze castings are of better quality than brass castings, but are more difficult to machine and more expensive.
The metallurgical technology of the manufacture of bronze is one of the most important milestones in the history of humanity, since it gave rise to the so-called Bronze Age. Bronze was the first alloy made voluntarily by humans: it was made by mixing copper ore (chalcopyrite, malachite, etc.) and tin (cassiterite) in a charcoal-fed furnace. The result of the combustion of coal, which oxidized to form carbon dioxide, produced the reduction of copper and tin ores to metals. The copper and tin that were melted, were alloyed between 5 and 10% by weight of tin.
Bronze is especially used in heat-conducting alloys, in electric batteries, and in the manufacture of valves, pipes, and plumbing joints. Some bronze alloys are used in sliding joints, such as bearings and rests, friction discs; and other applications where high resistance to corrosion is required, such as turbine runners or pump valves, among other machine elements. In some electrical applications it is used in springs.
Alpaca (Cu-Ni-Zn)
Alpacas or German silver are alloys of copper, nickel (Ni) and zinc (Zn), in a proportion of 50-70% copper, 13-25% nickel, and 13-25% zinc. Its properties vary continuously depending on the proportion of these elements in its composition, going from maximum hardness to minimum conductivity. These alloys have the property of repelling marine organisms (antifouling). If small amounts of aluminum or iron are added to these copper-nickel-zinc alloys, they constitute alloys that are characterized by their resistance to marine corrosion, which is why they are widely used in shipbuilding, mainly in condensers and pipes, as well as as in the manufacture of coins and electrical resistances.
Alpaca alloys have good corrosion resistance and good mechanical qualities. Its application covers telecommunications materials, plumbing and electrical instruments and accessories, such as taps, clamps, springs, connectors. It is also used in construction and hardware, for decorative elements and in the chemical and food industries, as well as tableware and goldsmith materials.
Monel is an alloy obtained directly from Canadian ores and has a composition of Cu=28-30%, Ni=66-67%, Fe=3-3.5%. This material is highly resistant to corrosive agents and high temperatures.
Another type of nickel silver is called platinoid, a white alloy composed of 60% copper, 14% nickel, 24% zinc and 1-2% tungsten.
Other alloys
Other copper alloys with technical applications are the following:
- Copper-cadmio (Cu-Cd)
- They are copper alloys with a small percentage of cadmium and have greater resistance than pure copper. They are used in air lines subject to strong mechanical requests such as catenaries and tram contact cables.
- Copper-chrome (Cu-Cr) and Copper-cromo-circonium (Cu-Cr-Zr)
- They have high electrical and thermal conductivity. They are used in resistance welding electrodes, collector rods, power contactors, steel equipment and conductor springs.
- Copper-hero-phosphorus (Cu-Fe-P)
- For the manufacture of elements that require good electrical conductivity and good thermal and mechanical properties are added to copper iron particles and phosphorus. These alloys are used in integrated circuits because they have good electrical conductivity, good mechanical properties and have high temperature resistance.
- Copper-aluminum (Cu-Al)
- Also known as aluminum bronzes and duraluminium, they contain at least 10% of aluminum. These alloys are very similar to gold and highly appreciated for artistic works. They have good mechanical properties and high corrosion resistance. They are also used for aircraft landing gears, in certain mechanical constructions.
- Copper-beril (Cu-Be)
- It is an alloy essentially made of copper. This alloy has important mechanical properties and high corrosion resistance. It is used to manufacture springs, plastic moulds, resistance welding electrodes and anti-deflagrating tools.
- Copper-plata (Cu-Ag) or silver copper
- It is a weak alloy due to its high copper content, which is characterized by a high hardness that allows it to withstand temperatures up to 226 °C, maintaining the electrical conductivity of copper.
- Constantán (Cu55Ni45)
- It is an alloy made up of 55 % copper and 45 % nickel. It is characterized by an electrical resistivity of 4,9•10−7Ω•m almost constant in a wide range of temperatures, with a temperature coefficient of 10−5K−1. It is used in the manufacture of thermocouples, extensiometric gauzes and coins.
- Manganina (Cu86Mn12Ni2)
- It is another alloy with a very low temperature coefficient and is used in extensiometric gauzes and high stability resistors. In addition, its thermoelectric contact potential with copper per Seebeck effect is very small (+0.6 mV/100 K). Its electric resistivity is about 4.9•10−7Ω•m and its temperature coefficient is 10−8K−1.
Some copper alloys have small percentages of sulfur and lead that improve the machinability of the alloy. Both lead and sulfur have very low solubility in copper, separating respectively as lead (Pb) and cuprous sulfide (Cu2S) at grain boundaries and facilitating chip breaking at the grain boundaries. machining processes, improving the machinability of the alloy.
Copper industrial processes
Copper mining
Native copper usually accompanies its minerals in pockets that emerge to the surface and are exploited in open pit mines. Copper is obtained from sulphide minerals (80%) and oxidized minerals (20%), the former are treated by a process called pyrometallurgy and the latter by another process called hydrometallurgy. The minerals are generally found in the upper layer oxidized (cuprite, melaconite), together with native copper in small quantities, which explains its millenary production since the metal could be easily extracted in pit furnaces. Next, below the water table, are the primary pyrites (sulfides) chalcocite (Cu2S) and covellin (CuS) and finally the secondary chalcopyrite (FeCuS2) whose exploitation is more profitable than the previous ones. Accompanying these minerals are others such as bornite (Cu5FeS4), gray copper and azurite and malachite carbonates that usually form important masses in copper mines. for being the way in which sulfides are usually altered.
The technology for obtaining copper is very well developed, although it is laborious due to the poor grade of minerals. Copper deposits generally contain very low concentrations of the metal. This is why many of the different production phases are aimed at removing impurities.
Copper metallurgy
Copper metallurgy depends on whether the mineral is in the form of sulfides or oxides (cuprous or cupric).
Pyrometallurgy
For sulfides, the route called pyrometallurgy is used to produce cathodes, which consists of the following process: Comminution of the mineral -> Concentration (float) -> furnace casting -> step to converters -> refine -> anode molding -> electrorefining -> cathode. The refining process produces cathodes with a 99.9% copper content. The cathodes are plates of one square meter and a weight of 55 kg.
Other components obtained from this process are iron (Fe) and sulfur (S), as well as very small amounts of silver (Ag) and gold (Au). Lead (Pb), arsenic (As) and mercury (Hg) are also extracted as impurities from the process.
As a general rule, a copper metallurgical facility that produces 300,000 t/year of anodes consumes 1,000,000 t/year of copper concentrate and produces 900,000 t/year of sulfuric acid and 300,000 t/year of slag as by-products.
Hydrometallurgy
When it comes to taking advantage of mineral residues, the small concentration of copper found in them is in the form of oxides and sulfides, and to recover this copper, the technology called hydrometallurgy is used, better known by its Anglo-Saxon nomenclature Sx- Ew.
The process that this technique follows is the following: Copper ore-> leach-> extraction-> electrolysis-> cathode
This technology is rarely used because almost all copper concentrates are forming sulfides, with estimated world production from waste recovery being around 15% of all copper produced.
Copper heat treatments
Copper and its alloys allow certain heat treatments for very specific purposes, the most common being annealing, refining and tempering.
Annealed hard copper is very good for cold operations such as: bending, stamping and drawing. Annealing is produced by heating copper or brass to a suitable temperature in a controlled atmosphere electric furnace, and then allowing it to cool in air. Care must be taken not to exceed the annealing temperature because then the copper burns and becomes brittle and becomes unusable.
Refining is a controlled process of oxidation followed by reduction. The objective of oxidation is to eliminate the impurities contained in the copper, volatilizing them or reducing them to slag. Next reduction is to improve the ductility and malleability of the material.
The heat treatments carried out on brass are mainly homogenization, recrystallization and stabilization annealing. Brasses with more than 35% Zn can be quenched to make them softer.
Bronzes are usually subjected to homogenization annealing treatments for casting alloys; and annealed against hardness and recrystallization for wrought alloys. The tempering of two-constituent element bronzes is analogous to the tempering of steel: it is heated to about 600 °C and cools rapidly. With this, it is possible to reduce the hardness of the material, contrary to what happens when tempering steel and some bronzes with more than two components.
Applications and uses of copper
Whether considering the quantity or the value of the metal used, the industrial use of copper is very high. It is an important material in many economic activities and has been considered a strategic resource in conflict situations.
Metallic copper
Copper is used both with a high level of purity, close to 100%, and alloyed with other elements. Pure copper is mainly used in the manufacture of electrical cables.
Electricity and telecommunications
Copper is the non-precious metal with the best electrical conductivity. This, together with its ductility and mechanical resistance, have made it the most widely used material to manufacture electrical cables, both for industrial and residential use. Copper conductors are also used in many electrical equipment such as generators, motors, and transformers. The main alternative to copper in these applications is aluminum.
Most telephone cables are also made of copper, which also provide Internet access. The main alternatives to copper for telecommunications are fiber optics and wireless systems. On the other hand, all computer and telecommunications equipment contains copper to a greater or lesser extent, for example in its integrated circuits, transformers and internal wiring.
Means of transportation
Copper is used in various components of cars and trucks, mainly radiators (thanks to its high thermal conductivity and resistance to corrosion), brakes and bearings, in addition of course to cables and electric motors. A small car contains a total of around 20 kg of copper, raising this figure to 45 kg for larger ones.
Trains also require large amounts of copper in their construction: 1 - 2 tons in traditional trains and up to 4 tons in high-speed trains. In addition, the catenaries contain about 10 tons of copper per kilometer on high-speed lines.
Finally, ship hulls often include copper and nickel alloys to reduce fouling by marine creatures.
Construction and ornamentation
A large part of the water transport networks are made of copper or brass, due to their resistance to corrosion and their anti-bacterial properties, lead pipes having fallen into disuse due to their harmful effects on health human. Compared to plastic pipes, copper pipes have the advantage that they do not burn in the event of a fire and therefore do not release potentially toxic fumes and gases.
Copper and, above all, bronze are also used as architectural elements and coverings on roofs, facades, doors and windows. Copper is also often used for doorknobs in public places, since its antibacterial properties prevent the spread of epidemics.
Two classic applications of bronze in construction and ornamentation are the making of statues and bells.
The construction sector currently consumes (2008) 26% of the world's copper production.
Coins
Since the beginning of the minting of coins in the Ancient Age, copper has been used as raw material, sometimes pure and, more often, in alloys such as bronze and cupronickel.
Examples of coins that include pure copper:
- The coins of one, two and five cents of euro are copper-coated steel. The US dollar penny is copper-coated zinc.
Examples of cupronickel coins:
- Inner disc of the currency of a euro and external part of the currency of two euros. Coins of US$ 25 and 50 cents. Spanish coins of 5, 10, 25, 50 and 200 pesetas coined since 1949.
Examples of coins made of other copper alloys:
- The coins of ten, twenty and fifty cents of euro are of Nordic gold, an alloy containing 89 % of copper. Argentinian coins of 5, 10, 25 and 50 cents in gold are 92 % copper and 8 % aluminum.
Other applications
Copper participates in the raw material of a large number of different and varied components of all types of machinery, such as bushings, bearings, trims, etc. It is part of the elements of costume jewellery, light bulbs and fluorescent tubes, boilermaking, electromagnets, coins, wind musical instruments, microwaves, heating and air conditioning systems. Copper, bronze and brass are suitable for galvanizing treatments to cover other metals.
Non-metallic copper
Copper(II) sulfate, also known as cupric sulfate, is the most industrially important copper compound and is used as a fertilizer and pesticide in agriculture, an algaecide in water purification, and as a wood preservative.
Copper sulfate is especially indicated to supply the main functions of copper in the plant, in the field of enzymes: ascorbic acid oxidases, polyphenol, cytochrome, etc. It is also part of the plastocyanin contained in chloroplasts and which participates in the electron transfer chain of photosynthesis. Its absorption is carried out through a metabolically active process. It is practically unaffected by competition from other cations but, on the contrary, affects other cations. This product can be applied to all types of crops and in any climate zone in greenhouses.
For the decoration of tiles and ceramics, glazes are made that provide a metallic shine of different colors. To decorate the piece once fired and glazed, mixtures of copper oxides and other materials are applied and then the piece is fired again at a lower temperature. By mixing other materials with the copper oxides, different shades can be obtained. ceramic decorations, metallic films of silver and copper are also used in colloidal mixtures (colloidal copper) of ceramic glazes that provide tones similar to the metallic iridescence of gold or copper.
A pigment widely used in painting for green tones is verdigris, also known in this field as verdigris, which consists of a mixture formed mainly by copper acetates, which provides greenish or bluish tones.
Industrial applications of copper
Casting: blister and anodes
Blister copper, also called blister or anode, has a purity between 98 and 99.5%, and its main application is the electrolytic manufacture of copper cathodes, whose purity reaches 99.99%. It can also be used to synthesize copper sulfate and other chemicals. Its main application is its transformation into copper anodes.
The intermediate step in the transformation of blister copper into copper cathodes is the production of copper anodes, with about 99.6% purity. A copper anode has approximate dimensions of 100x125 cm, a thickness of 5 cm and an approximate weight of 350 kg.
Refinery: cathodes
Copper cathode is the ideal raw material for the production of high-specification copper rod. It is a product, with a copper content greater than 99.99%, resulting from the electrolytic refining of copper anodes. Its quality is within the Cu-CATH-01 denomination under the EN 1978:1998 standard. It comes in corrugated and strapping packages, whose sheet has dimensions of 980x930 mm and a thickness of 7 mm with an approximate weight of 47 kg. Its fundamental use is the production of high-quality copper wire rod, although it is also used for the production of other highly demanding semi-transformed products.
Smelting and refining by-products
After the process of making copper anode and copper cathode, the following by-products are obtained: Sulfuric acid. Granulated slag. electrolytic sludge. Nickel sulphate. And so
Wire Rod
Copper wire rod is a product resulting from cathode transformation in continuous casting. Its production process is carried out according to ASTM B49-92 and EN 1977 standards.
The essential characteristics of the wire rod produced by the Atlantic-copper company are:
- Diameter and tolerance: 8 mm ± 0.4 mm. What: 99.97 % min. Oxygen: 200 ppm. Electrical conductivity: /2005 101 % (IACS. Spiral elongation test: 450 m (200 °C)
The wire rod is sold in coils strapped on a wooden pallet and protected with a plastic cover. Whose dimensions are: coil weight 5000 kg, outer diameter 1785 mm, inner diameter 1150 mm and height 900 mm. The applications of the wire rod are for the manufacture of electrical cables that require high quality, whether they are enameled or multi-wire with diameters of 0.15/0.20 mm.
Bare copper wire
Bare copper wire is produced from wire rod and through a grinding process and with an annealing furnace. Bare wire formed by an electrolytic copper wire in three tempers, hard, semi-hard and soft, is obtained and is used for electrical purposes. It is produced in a range of diameters from 1 mm to 8 mm and in coils that can weigh in the order of 2,250 kg.. This wire is used in overhead power distribution lines, in substation neutrals, equipment and system ground connections, and to manufacture flat, enameled, and multi-wire wires that can have a diameter of 0.25/0.22 mm. It is made from high purity copper with a minimum Cu content of 99.9%. This type of wire has a high conductivity, ductility and mechanical resistance as well as great resistance to corrosion in brackish environments.
Wire Drawing
Wire drawing is the process of thinning copper through the mechanical stretching that is exerted on it when starting from 6 or 8 mm diameter wire rod with the aim of producing flexible electrical cables with the required section. An electrical cable is made up of several wires that, through an extrusion process, are applied to the outer insulation with a PVC or polyethylene plastic compound. Generally, the input caliber is 6 to 8 mm, to later thin it to the required diameter. As wire drawing is a continuous process, different coils or rolls are formed that are cut to the lengths required or established by the standards and are duly labeled with the corresponding technical data of the cable.
Shielding is the covering of a properly insulated central conductor by several copper conductor wires, which intertwine around them to form a screen. When it is necessary to insulate a conductive wire by means of enamelling, a layer of varnish (polyesterimide) is applied to it. These mixtures of resins are used to coat the metallic conductor, being isolated from the environment that surrounds it and thus managing to conduct the electrical flow without problems.
Tubes
A tube is a hollow product, normally round in section, which has a continuous periphery and is used in gas fittings, plumbing and mechanical systems for the transport of liquids or gases. Copper pipes are widely used in residential, commercial and industrial buildings.
To manufacture tubes, generally, a mixture of refined copper and quality-controlled scrap is used, which is melted in a furnace. Ingots known as "billets" are obtained from copper casting, which are cylindrical in shape, about 300 mm in diameter and 8 m long and weigh about 5 tons. Seamless tubes are manufactured from these ingots by the following operations:
- Cut the ingots into pieces of about 700 mm long.
- Tunnel furnace heating at between 800 and 900 °C.
- Extrusion at high temperature, for which a piece or large diameter pretube with very thick walls is obtained in a single passage. The machine in which this operation is performed is called "extrusive" and consists of a "mandril" that tightens the hot lingote until it passes through a calibrated matrix. During extrusion, copper suffers from superficial oxidation that may harm subsequent operations.
- Cold lamination, which reduces the thickness of the tube wall, keeping its circular section. It is done by passing the pretube, with a mandril inserted, between two cylinders that rotate in the opposite sense and that have a movement of vaivén in the longitudinal sense.
- Cold trefilling, which stretches the tube and reduces its diameter to the different commercial specifications. It is done in a machine called "Bull Block" in which the tube is forced to pass through several external matrices and an internal caliber called "floating rail".
- Recocido, thermal treatment that reclaims copper and allows you to recover the plasticity lost throughout the previous operations.
- Finished. The coated tube can be applied to various types of finish depending on the application, for example an external coating of protection or a very smooth internal finish.
- Quality control. A test used frequently to detect imperfections in copper tubes is that of electromagnetic induction by Foucault currents.
- Packaging, which varies according to the type of tube. While the harvests or soft temples need packaging that protects them from deformations during the transport, the cold-washed tubes of hard temper are simply packed into tied. There is no need to protect atmospheric agents.
Lamination
One of the fundamental properties of copper is its malleability, which makes it possible to produce all kinds of sheets from very small thicknesses, both in the form of a continuous roll and in sheets of various sizes, using the appropriate lamination facilities.
Casting parts
Pure copper is not very suitable for casting because it produces galleo. This phenomenon consists of the oxygen in the air being absorbed on the copper at high temperatures and forming bubbles; when the metal later cools, the oxygen in the bubbles is released, leaving microscopic voids on the surface of the castings.
Its alloys do allow parts to be manufactured by any of the casting processes that exist depending on the type of part and the quantity that has to be produced. The most common casting methods are by casting and by centrifugation.
The process of manufacturing parts, commonly metal but also plastic, is called casting by molding, consisting of melting a material and introducing it into a cavity, called a mold, where it solidifies. The traditional process is sand casting, as sand is a refractory material that is abundant in nature and which, mixed with clay, acquires cohesion and moldability without losing the permeability that allows the gases to evacuate from the mold while the molten metal is poured.
The centrifugal casting process consists of depositing a layer of liquid casting in a revolving mold rotating at high speed and rapidly solidifying the metal by means of continuous cooling of the mold or shell. The applications of this type of foundry are very varied.
Forged
The hot forging of a part consists of inserting a metal bar into a mold, heating it to the appropriate temperature and forcing it to plastically deform until it adopts the shape of the mold. The advantage of hot forging is that the mechanical power that must be supplied by the press for plastic deformation is reduced.
Copper products and their alloys meet very good conditions to produce parts by hot stamping processes, allowing the design of extremely complex parts thanks to the great ductility of the material and the low resistance to deformation it opposes, thus providing a long life to the matrices. A copper alloy is hot-forgeable if there is a sufficiently wide temperature range in which ductility and resistance to deformation are acceptable. This range of temperatures depends on its chemical composition, which is influenced by the added elements and impurities.
Machining
Copper parts or their alloys that are going to be subjected to chip removal machining work have a small contribution of lead and sulfur in their chemical composition that causes a better fracture of the cut chips.
Currently (2008) the machining of copper components is carried out under the concept of rapid dry machining with the air-cooled tool if necessary. This type of fast machining is characterized by the fact that the machine heads rotate at very high speeds, achieving high cutting speeds in small diameter tools.
Likewise, the tools used are usually solid carbide, with special coatings that make it possible to work with very high cutting feeds. The coatings and materials of these tools are highly resistant to wear, they can work at high temperatures, which is why refrigeration is often not necessary, they have a very low coefficient of friction and achieve very fine and precise surface finishes.
Welding
Two different types of solder are used to solder joints made of copper or its alloys: soldering and brazing.
Soft soldering is one that is carried out at a temperature of about 200 °C and is used to join the components of printed and electronic circuits. Tin soldering irons are used and the filler material is an alloy of tin and lead in the form of coiled wire with deoxidizing resin in its core. It is a low resistance solder and serves to ensure the continuity of the electrical current through the circuit.
The welding of water and gas pipes carried out by plumbers are of various types depending on the materials to be joined and the tightness that they want to achieve from the weld. Currently, most pipes in plumbing fixtures are made of copper, although other materials are sometimes used as well.
Copper pipe brazing is done with gas torches that provide the flame to melt the solder. Torch fuel can be butane or propane.
Copper is also used as a binder in plumbing brazing, used for gas pipes and complex hot water piping. An alternative metal for this application is silver.
Boilermaking
Boilermaking is called a professional specialty in the metal manufacturing branch whose main function is the construction of tanks suitable for the storage and transport of solids in the form of grains or aggregates, liquids and gas, as well as all types of shipbuilding and metal structures. Thanks to the excellent thermal conductivity that copper sheet has, it is used to make stills, boilers, coils, covers, etc.
Crafting
Drawing is the name given to the cold forming process by which a disc or cut pieces are transformed, depending on the material, into hollow pieces, and even starting from previously drawn pieces, stretching them to a smaller section with greater height.
The objective is to obtain a hollow part according to the shape defined by the drawing die that is used, through the pressure exerted by the press. The drawing die is also known as a mold.
This is a sheet metal forming process by plastic deformation during which the sheet simultaneously undergoes transformations by stretching and upsetting, producing variations in its thickness. Hydraulic presses are almost exclusively used for drawing.
Copper sheet and its alloys have very good properties to be cold formed. Deep drawing is a good process for manufacturing parts with complex surfaces and high dimensional requirements in thin sheet metal, successfully replacing parts traditionally manufactured by casting and machining.
Stamping
The name of stamping is the mechanical operation that is carried out to engrave a drawing or a legend on the flat surface of a piece that is generally sheet metal. Copper sheets and their alloys meet very good conditions to carry out all kinds of engravings on them.
The key elements of stamping are a press that can be mechanical, pneumatic or hydraulic; of very varied size, shape and power, and a matrix called stamp or die, where the drawing to be minted on the plate is engraved, and that when it is struck sharply on it, it is engraved.
The stamping of metals is done by pressure or impact, where the sheet adapts to the shape of the mold. Stamping is one of the easiest machining tasks that exists, and allows a high level of process automation when it comes to making large numbers of parts.
The stamping can be done cold or hot, the stamping of hot parts is called forging, and it has a different operation from the cold stamping that is generally done on sheets. Steel, aluminum, silver, brass and gold sheets are the most suitable for stamping. One of the best-known stamping tasks is the one that stamps the faces of the coins in the minting process.
Die cut
Die-cutting is the mechanical operation that is performed to produce sheet metal parts or where it is necessary to make various holes in them. To carry out this task, everything from simple manual drive mechanisms to sophisticated high-powered mechanical presses are used.
The basic elements of a die-cutting press are made up of the die that has the shape and external dimensions of the piece or of the holes to be made, and the cutting die through which the die is inserted when it is energetically driven due to the power provided by the press through an eccentric drive that it has and that provides a dry and forceful blow on the sheet, producing a clean cut from it.
Depending on the work that has to be done, this is how the presses are designed and built. There are simple and progressive dies where the sheet, which is in the form of large rolls, advances automatically, causing continuous work, and requiring no other care than changing the roll of sheet metal when it is finished and removing the stamped pieces as well as monitoring the quality of the cut they make.
When the cut deteriorates due to wear of the die and the matrix, they are disassembled from the machine and ground on a flat grinder, establishing a new cut. A die and die allow many regrinds until they are completely worn out.
There are die-cutters that work with a head where several dies of different sizes can be inserted and a wide table where the sheet to be machined is placed. This table is activated by CNC and moves along and across it at high speed, producing parts quickly and accurately.
Copper toxicity
The mechanisms underlying the effects of Cu poisoning in humans are not well understood. Cu is a transition metal that, like the rest of this type of metal (except Zn), has unpaired electrons in its outer orbitals. For this reason, these metals can be considered free radicals.
Human Exposure
Copper can be found in many kinds of food, in drinking water, and in the air. Because we absorb an eminent amount of copper every day through eating, drinking and breathing. The absorption of copper is necessary, because copper is a trace element that is essential for human health. Although humans can handle proportionally high concentrations of copper, a large amount of this mineral in our bodies can cause health problems.
Copper concentrations in the air are usually quite low, so exposure to copper through respiration is negligible. But people who live near smelters that process copper ore into metal can experience this kind of exposure.
People who live in homes that still have copper plumbing are exposed to higher levels of copper than most people, because copper is released into their water through corrosion of the pipes.
Environmental exposure
More and more copper ends up in the environment. Rivers are depositing mud on their banks that is contaminated with copper, due to the discharge of copper-contaminated sewage. Copper enters the air, mostly through the release during the combustion of fuel. The copper in the air will remain for an eminent period of time, before settling down when it starts to rain. This will mostly end up in soils, as a result soils can also contain large amounts of copper after it is deposited from the air.
Copper can be released into the environment by both human activities and natural processes. Examples of natural sources are dust storms, decomposition of vegetation, forest fires and marine aerosols. Copper is often found near mines, industrial settlements, landfills, and waste sites.
When copper ends up in the soil it is strongly bound to organic matter and minerals.
Copper does not break down in the environment and therefore can accumulate in plants and animals when it is found in soils. In copper-rich soil only a small number of plants can live. Copper can seriously influence the processing of certain agricultural lands, depending on the acidity of the soil and the presence of organic matter. Despite this copper-containing dung is still used.
Copper can disrupt activity in the soil, its negative influence on the activity of microorganisms and earthworms. The decomposition of organic matter can decrease due to this.
When farm soils are contaminated with copper, animals can absorb concentrations of copper that harm their health. Mainly sheep are greatly affected by copper poisoning, because the effects of copper are manifested at low concentrations.
Acute toxicity
Although reference chemical works indicate that copper salts are toxic, in practice this is only true when the solutions are used uncontrolled, for suicidal purposes or as a topical treatment of burnt areas serious. When copper sulfate, also known as blue stone or blue vitriol, is ingested in amounts on the order of grams, nausea, vomiting, diarrhea, sweating, intravascular hemolysis, and possible renal failure occur; rarely, seizures, coma, and death are also seen. When carbonated water or citrus juices that have been in contact with copper containers, pipes, faucets or valves are drunk, irritation of the gastrointestinal tract can occur, which is rarely serious. These types of drinks are acidic enough to dissolve irritating levels of copper. There is one report of corneal ulcers and skin irritation, with low other toxicity, in a copper miner who fell into an electrolytic bath, although the cause may have been acidity rather than copper. In some cases where copper salts were used for the treatment of burns, elevated serum copper concentrations and toxic manifestations were observed. Inhalation of copper salt dusts, fumes, or mists may cause nasal and mucosal congestion, and ulceration with perforation of the nasal septum. The fumes released during the heating of metallic copper can cause fever, nausea, gastralgia, and diarrhea.
Chronic toxicity
Chronic toxic effects attributable to copper only seem to exist in people who have inherited a specific pair of autosomal recessive genes and who, as a consequence, develop hepatolenticular degeneration (Wilson's disease). It is a rare disease. Most of the daily food consumed by man contains from 2 to 5 mg of copper, which is practically not retained in the body.
The body content of copper in an adult person is 100 to 150 mg and is almost constant. In normal individuals (without Wilson's disease), nearly all copper is present as an integral and functional part of a dozen protein and enzyme systems, including cytochrome oxidase, dopa oxidase, and serum ceruloplasmin.
In people who eat large amounts of oysters or shellfish, liver, mushrooms, nuts and chocolate, all foods rich in copper, or in miners who work and eat for 20 years or more in an environment loaded with a 1 or 2% copper mineral dust, concentrations up to 10 times higher than normal can be observed.
However, no case of primary chronic copper toxicity (clearly defined from observations of patients with inherited chronic copper toxicosis 'Wilson's disease' as dysfunction and lesions) has yet been reported. liver, central nervous system, kidney, bone, and eye structures) except in people with Wilson's disease. However, excessive copper deposits found in the liver of patients with primary biliary cirrhosis, cholestasis, and infantile cirrhosis from India may contribute to the severity of the liver disease characteristic of these conditions.
Mechanisms of toxicosis
The mechanisms underlying the effects of Cu poisoning in humans are not well understood. Cu is a transition metal that, like the rest of this type of metal (except Zn), has unpaired electrons in its outer orbitals. For this reason, these metals can be considered free radicals. Cu, like iron, can participate in Fenton (1) and Häber-Weiss (2) type reactions producing ROS:
- Cu++ H2O2→2++ OH• + OH-
- Cu2++ O2-• →++ O2
Cu+ salts react with H2O2 more efficiently than Fe2+. Thus, the main mechanism of copper-mediated toxicosis may lie in its ability to cause ROS overproduction and subsequent pro-oxidative damage to lipids, nucleic acids, and proteins.
Copper has important effects as a cytotoxic and genotoxic agent, playing an important role in the etiopathogenesis of neoplasms. This last mechanism consists of damaging the molecular structure of DNA indirectly (ROS) or directly by forming complexes with functional groups of the nitrogenous bases that modify them by introducing mutations, or hindering the repair process.
It is believed that one of the ways in which Cu ions exert their toxic effect is by producing an increase in oxidative stress in multiple tissues of the organism.
Recycled
Copper is one of the few materials that does not degrade or lose its chemical or physical properties in the recycling process. It can be recycled an unlimited number of times without losing its properties, making it impossible to distinguish whether a copper object is It is made from primary or recycled sources. This means that copper has been, since Antiquity, one of the most recycled materials.
Recycling provides a critical part of total metallic copper needs. It is estimated that in 2004, 9% of world demand was met by recycling old copper objects. If you also consider "recycling" the remelting of waste from the mineral refining process, the percentage of recycled copper amounts to 34% in the world and up to 41% in the European Union.
Recycling copper does not require as much energy as mining it. Although recycling requires collecting, sorting, and smelting metal objects, the amount of energy needed to recycle copper is only about 25% of that required to convert copper ore into metal.
The effectiveness of the recycling system depends on technological factors such as product design, economic factors such as the price of copper, and social factors such as public awareness of sustainable development. Another key factor is legislation. Currently, there are more than 140 national and international laws, regulations, directives and guides that try to promote the responsible management of the end of life cycle of products that contain copper, such as household appliances, telephones and vehicles.
In the European Union, Directive 2002/96/EC on waste electrical and electronic equipment (WEEE, or WEEE in English Waste Electrical and Electronic Equipment) favors a waste minimization policy, which includes a mandatory and drastic reduction of industrial and household waste, and incentives for producers who produce less waste. The goal of this initiative was to recycle 4 kilos per inhabitant per year by the end of 2006.
An example of massive copper recycling was the replacement of the national currencies of twelve European countries by the euro in 2002, the largest currency change in history. Some 260,000 tons of coins were removed from circulation, containing approximately 147,496 tons of copper, which were melted down and recycled for use in a wide range of products, from new coins to different industrial products.
Production and trade
Mining production
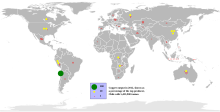
World copper production during 2019 reached a total of 20 million metric tons of fine copper. The main producing country is Chile, with almost a third of the total, followed by Peru and China:
Of the ten largest copper mines in the world, three are in Chile (Escondida, Codelco Norte, Collahuasi, El Teniente and Los Pelambres), two in Indonesia, one in the United States, one in Russia and one in Peru (Antamina, Toquepala, Cuajone, Cerro verde).
|
A different picture emerges when analyzing the companies that manage copper mines. According to a report by the specialized magazine illuminem, the main producers are companies based in Great Britain followed by companies incorporated in Chile, the United States and Mexico, while China ranks fifth in economic control of copper mines.
Reservations
According to information provided in the annual report of the United States Geological Survey (USGS), estimates indicate that known copper reserves in 2011 worldwide would reach 690 million metric tons of fine copper. And according to USGS estimates, in Chile there would be around 190 million economically exploitable tons, equivalent to 28% of the total world mineral reserves; followed by Peru with 90 million economically exploitable tons, equivalent to 13% of the total world mineral reserves:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Trade and consumption
Copper is the third most used metal in the world, behind iron and aluminum. There is an important world trade in copper that moves about 30,000 million dollars annually.
The three main copper markets are the LME in London, the COMEX in New York and the Shanghai Metal Exchange. These markets set the price of copper and futures contracts on the metal on a daily basis. of $4.00/lb reached in 2006 and 2008. The sharp rise in copper prices since 2004, mainly due to increased demand from China and other emerging economies, has led to a wave of theft of copper objects (especially all cables) all over the world, with the consequent risks for the electrical infrastructure.
| Rank | State | Consumption of refined copper (in million ton/year) |
|---|---|---|
| 1 | European Union | 4.32 |
| 2 | China | 3.67 |
| 3 | United States | 2,13 |
| 4 | Japan | 1.28 |
| 5 | South Korea | 0.81 |
| 6 | Russia | 0.68 |
| 7 | Taiwan | 0.64 |
| 8 | India | 0.44 |
| 9 | Brazil | 0.34 |
| 10 | Mexico | 0.30 |
Source: World Copper Factbook 2007
The main producers of copper ore are also the main exporters of both ore and refined copper and derivatives. The main importers are the industrialized countries: Japan, China, India, South Korea and Germany for ore and the United States, Germany, China, Italy and Taiwan for refining.
Evolution of copper price
centavos of dollar per pound of copper (November 2008=100)

Source: Sociedad Nacional Minera de Chile
Contenido relacionado
Echolocation
LEDs
Amstrad