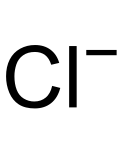Chloride
The chlorides are anions derived from hydrogen chloride and are compounds that have a chlorine atom in the -1 formal oxidation state. Therefore they correspond to the lowest oxidation state of this element since it has completed the valence shell with eight electrons.
Inorganic chlorides
General characteristics
Inorganic chlorides contain the anion Cl− and are therefore salts of hydrochloric acid (HCl). They are usually colorless solid substances with a high melting point.
In some cases the bond with the metal may have a certain covalent character. This is noted for example in mercury(II) chloride (HgCl2) which sublimes at fairly low temperatures. This is why this salt was formerly known by the name of "sublimate".
Iron(III) chloride (FeCl3) also shows some covalent character. Thus, it can be extracted from a solution with a high concentration of chloride with ether and without the presence of sublime crystallization water at high temperatures.
Most of the chlorides with the exception mainly of mercury(I) chloride (Hg2Cl2), silver chloride (AgCl) and chloride Thallium(I) (TlCl) are quite soluble in water.
In the presence of strong oxidants (permanganate, bismuthate, hydrogen peroxide, hypochlorite, etc.) chlorides can be oxidized to elemental chlorine. This oxidation can also be carried out by electrolysis. In fact, the electrolysis of sodium chloride in solution is the most used method to obtain this element in addition to sodium hydroxide.
Summary
Chlorides can be obtained by reacting a base (oxide, hydroxide, carbonate, etc.) and hydrochloric acid.
Some less noble metals also react directly with hydrochloric acid to give elemental hydrogen and the corresponding chloride. The reaction with zinc, for example, would be the following:
Zn + 2 HCl -> ZnCl2 + H2
It is also possible to react directly from the elements, although in many cases it is very violent.
Presence
The best known chloride is sea salt which is present in seawater at a concentration of approximately 3-3.5%. Therefore the oceans represent a practically inexhaustible source of chloride.
Analytics
Soluble chlorides precipitate from an acid solution in the presence of silver nitrate, forming a pale silver chloride solid. The precipitate dissolves in ammonia and precipitates again by acidifying with nitric acid.
Properties
Solubility in water
Solubility of anhydrous salts in water at room temperature (20 to 25 °C) in g/100g H
2 O (considered chlorides: AlCl
3, SbCl
3, BaCl
2 , BeCl
2, CdCl
2, CaCl
2, CsCl, CoCl
2, CuCl
2, AuCl
3, InCl
3, FeCl
3, LaCl
3, PbCl
2, LiCl, MgCl
2, MnCl
2, HgCl
2, NdCl
3, NiCl
2, PtCl
4, KCl, PrCl
3, RaCl
2, RbCl, SmCl
3, AgCl, NaCl, SrCl
2, TlCl, YCl
3, ZnCl
2)
| H | He | ||||||||||||||||
| Li 84,5 | Be 71.5 | B | C | N | O | F | Ne | ||||||||||
| Na 36 | Mg 56 | Al 45.1 | Yeah. | P | S | Cl | Ar | ||||||||||
| K 35.5 | Ca 81.3 | Sc | Ti | V | Cr | Mn 77.3 | Fe 91.2 | Co 56.2 | Ni 67.5 | Cu 75.7 | Zn 408 | Ga | Ge | As | Separate | Br | Kr |
| Rb 93.9 | Mr. 54.7 | And 75.1 | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag 0,00019 | Cd 120 | In 195.1 | Sn | Sb 987 | You | I | Xe |
| Cs 191 | Ba 37 | ♪ | Hf | Ta | W | Re | You | Go | Pt 142 | Au 68 | Hg 7.31 | Tl 0.33 | Pb 1,088 | Bi | Po | At | Rn |
| Fr | Ra 24,5 | ** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| ♪ | La 95.7 | Ce | Pr 96.1 | Nd 100 | Pm | Sm 93.8 | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||
| ** | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | That's it. | Fm | Md | No. | Lr | ||
Density
Density of salts in g.cm-3 (shorts considered: AcCl
3, AlCl
3, AmCl
3, SbCl
3, AsCl
3, BaCl
2, BeCl
2, BiCl
3, CdCl
2, CaCl
2, CeCl
3, CsCl, CrCl
3, CoCl
2, CuCl
2, ErCl
3, EuCl
2, GdCl
3, GaCl
3, GeCl
4, AuCl
3, HoCl
3, InCl
3, ICl
3, IrCl
3, FeCl
2, LaCl
3, PbCl
2, LiCl, LuCl
3, MgCl
2, MnCl
2, HgCl
2, MoCl
2, NdCl
3, NiCl
2, NbCl
5, NCl
3, PdCl
2, PCl
3, PtCl
2, PuCl
3, KCl, PrCl
3, RaCl
2, ReCl
3, RhCl
3, RbCl, RuCl
3, SmCl
3, ScCl
3, SeCl
4, AgCl, NaCl, SrCl
2, SCl
2, TaCl
5, TeCl
2, TbCl
3, TlCl, ThCl
4, SnCl
2, TiCl
2, WCl
6, UCl
3, VCl
2, YbCl
2, YCl
3, ZEmelyCl
2, ZrCl
2)
| H | He | ||||||||||||||||
| Li 2.07 | Be 1.9 | B | C | N 1.653 | O | F | Ne | ||||||||||
| Na 2,17 | Mg 2.325 | Al 2.48 | Yeah. | P 1.574 | S 1.62 | Cl | Ar | ||||||||||
| K 1,988 | Ca 2,15 | Sc 2.4 | Ti 3.13 | V 3,23 | Cr 2.76 | Mn 2,977 | Fe 3.16 | Co 3,36 | Ni 3.51 | Cu 3.4 | Zn 2.907 | Ga 2.47 | Ge 1.88 | As 2,15 | Separate 2.6 | Br | Kr |
| Rb 2.76 | Mr. 3,052 | And 2.61 | Zr 3.16 | Nb 2.78 | Mo 3.71 | Tc | Ru 3.1 | Rh 5,38 | Pd 4 | Ag 5,56 | Cd 4,088 | In 4 | Sn 3.9 | Sb 3.14 | You 6.9 | I 3.2 | Xe |
| Cs 3,988 | Ba 3.9 | ♪ | Hf | Ta 3.68 | W 3,52 | Re 4.81 | You | Go 5.3 | Pt 6 | Au 4.7 | Hg 5.6 | Tl 7 | Pb 5,98 | Bi 4.75 | Po | At | Rn |
| Fr | Ra 4.9 | ** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| ♪ | La 3,84 | Ce 3,97 | Pr 4 | Nd 4,13 | Pm | Sm 4.46 | Eu 4.9 | Gd 4.52 | Tb 4.35 | Dy | Ho 3.7 | Er 4,1 | Tm | Yb 5,27 | Lu 3,98 | ||
| ** | Ac 4.81 | Th 4.59 | Pa | U 5,51 | Np | Pu 5,71 | Am 5,87 | Cm | Bk | Cf | That's it. | Fm | Md | No. | Lr | ||
Organic chlorides
General characteristics
In organic chlorides, chlorine is directly attached to a carbon atom. The bond is covalent although due to the difference in electronegativity between the two elements it is strongly polarized. For this reason chlorine can be substituted in many cases in nucleophilic substitution reactions.
Organic chlorides are less flammable than the corresponding hydrocarbons. They are also often more toxic. Some chloroalkanes such as dichloromethane are important as solvents. Insecticides like lindane or DDT are also organic chlorides. Also in this group are chlorodibenzodioxins that have become famous and feared by the Seveso accident.
Summary
The chlorides of aliphatic compounds can be obtained by direct reaction of the substance with elemental chlorine. The reaction is usually violent and takes place through a non-selective radical mechanism.
Selectivity can be increased by using N-chlorosucinimide instead of elemental chlorine.
Usually it is more advisable to transform another functional group into chloride. Thus the hydroxy groups can be replaced by chloride by applying hydrochloric acid (possibly in the presence of zinc chloride as a catalyst), thienyl chloride, phosphorus chloride, etc.
They can also be obtained by adding chlorine or hydrochloric acid to multiple bonds of alkenes or alkynes. Hydrochloric addition gives mainly the Markownikov product (with the chlorine attached to the more substituted carbon) under polar conditions and the anti-Markownikov product (with the chloride on the less substituted carbon) under radical conditions.
Aromatic chlorides are usually finally obtained by direct chlorination in an electrophilic substitution reaction in the presence of a Lewis acid as a catalyst.
Analytics
A) Beilstein test:
To determine the presence of chloride in an organic compound, heat a copper wire in a blue flame from a Bunsen burner until no marked coloration is noted. Then it is put in contact with the organic compound and the compound with the wire is introduced into the flame. A bluish-green coloration indicates the presence of chloride.
Contraindications: Other halides and some amines can give the same reaction.
B) Transformation into inorganic chloride
A small sample is heated in a test tube with a small amount (a few milligrams) of sodium metal (CAUTION, EXPLOSIVE REACTIONS MAY OCCUR) until the tube is red hot. Then the tube is poured into a container with water, acidified with nitric acid and the chloride is precipitated with silver nitrate (AgNO3) performing the other tests as described in the case of chlorides. inorganic.
Biochemistry
Few organic chlorides exist in nature. Therefore organic chlorides usually have poor biodegradability and remain for years in the environment. Due to their hydrophobic nature, they accumulate in fats, especially in the last links of the food chain, and can cause health problems there, which makes chlorides very important in people's health.
Contenido relacionado
Maltase
Antioxidant
Polypeptide