Cerebellum
The cerebellum is a region of the brain whose main function is to integrate the sensory and motor pathways. There is a large number of nerve bundles that connect it with other brain structures and the spinal cord. The cerebellum integrates all the information received to specify and control the orders that the cerebral cortex sends to the locomotor system through the motor pathways. It is the regulator of physiological tremor.
For this reason, lesions at the level of the cerebellum do not usually cause paralysis, but they do cause disorders related to the execution of precise movements, maintenance of balance, posture and motor learning. Early studies by physiologists in the 18th century indicated that those patients with cerebellar damage showed motor coordination and movement problems. During the XIX century the first experiments began to be carried out functional, causing cerebellar lesions or ablations in animals. Physiologists noted that such injuries led to awkward, awkward movements, incoordination, and muscle weakness. These observations and studies led to the conclusion that the cerebellum was an organ in charge of motor control. However, modern research has shown that the cerebellum has a broader role, thus being related to certain cognitive functions such as attention. and the processing of language, music, learning, and other temporary sensory stimuli.
It was first described by Herophilus in the 4th century BC. c.
General characteristics
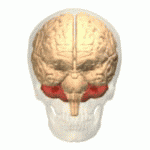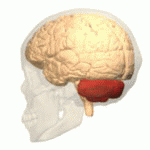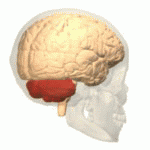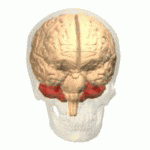
The cerebellum is an unpaired and a half organ, located in the posterior cranial fossa, dorsal to the brainstem and inferior to the occipital and ocular lobe.
Development
Like the rest of the central nervous system and the skin, the cerebellum is derived from the ectodermal layer of the trilaminar germ disc.
During the earliest phases of embryonic development, the cephalic third of the neural tube presents three dilations (primary brain vesicles) which allows us to divide it into three distinct segments: forebrain, midbrain, and rhombencephalon. The rhombencephalon is the most caudal segment, and when the embryo is 5 weeks old it divides into two portions: the metencephalon, and the myelencephalon. The metencephalon is the most cephalic portion and will give rise to the pons (pons) and the cerebellum, while the medulla oblongata (medulla oblongata) will originate from the myelencephalon. The limit between these two portions is marked by the pontine curvature.
Like all structures derived from the neural tube, the metencephalon is made up of alar and basal plates separated by the limiting sulcus. The alar plates contain sensory nuclei that are divided into three groups: the lateral somatic afferent group, the special visceral afferent group, and the general visceral afferent group. The basal plates contain motor nuclei that are divided into three groups: the medial somatic efferent group, the special visceral efferent group, and the general visceral efferent group.
The dorsolateral portions of the wing plates incurve medially to form the rhombic lips. In the caudal part of the midbrain, the rhombic lips are widely separated, but in the cephalic part they approach the midline. As the pontine fold deepens, the rhombic lips are compressed in a cephalocaudal direction and form the cerebellar plaque. At 12 weeks of development, the existence of three portions can be seen in the cerebellar plate: the vermis, in the midline, and two hemispheres, on both sides. Soon after, a transverse fissure separates the nodule from the rest of the vermis and the flocs from the rest of the hemispheres.
Initially, the cerebellar plate is composed of three layers, which from deep to superficial are: neuroepithelial layer, mantle layer, and marginal layer. At approximately 12 weeks of development, some cells originating in the neuroepithelial layer migrate to the more superficial zone of the marginal layer. These cells retain the ability to divide and begin to proliferate on the surface where they eventually form the outer granular layer. In the 6-month-old embryo, the outer granular layer begins to differentiate into various cell types that migrate inward to pass between the Purkinje cells and give rise to the inner granular layer. The outer granular layer ends up being devoid of cells and gives rise to the molecular layer. Basket cells and stellate cells arise from cells that proliferate in the white matter (marginal layer).
The deep cerebellar nuclei, such as the dentate nucleus, are in their final position before birth while the cerebellar cortex reaches its full development after birth.
- Origin and characteristics of the parent cells
Contrary to the classical anatomical idea; the adult cerebellum does not arise solely from the metencephalon. Studies by Hallonet and Nicole M. Le Douarin in the early 1990s showed that cerebellar progenitor cells come from the caudal region of the midsensephalon and the rostral region of the metencephalon. To show this, they created different chicken chimeras (Gallus gullus) and quail (Cournir coturnir juponica), with grafts of the metencephalic and mesensephalic regions of interest. Because quail cells present an interphase nucleus with condensed heterochromatin, these cells are easily distinguishable from chicken cells after Feulgen staining (stains DNA) (See external link). Using this methodology, Hallonet and Le Douarin showed that the mediorostral cells of the adult cerebellum originate from the caudal area of the midbrain, while the rest of the cerebellar progenitor cells originate from the rostral area of the metencephalon. The authors emphasize the strictly metencephalic origin of the outer granule layer (EGL) cells, which will give rise to granule cells at later stages of development. The other cells of the cerebellum (Purkinje cells, for example) come from the mesencephalic and metencephalic vesicles.
Gao and Hatten wanted to show the potential of progenitor cells from the external granular layer (EGL) and compare it with the potential of progenitor cells from the ventral zone (VZ). To do this, they isolated precursor cells from these areas from E13 mice, then implanted them into the outer granular layer of postnatal mice and observed the cell types into which these cells differentiated. The progenitor cells of the outer granule layer (EGL) were observed to be unipotent, producing only granule cells. In contrast, cells from the ventral zone differentiated into Purkinje neurons, interneurons, astroglia, and granule cells, which evidences the restrictions that occur during development depending on the spatial and temporal contexts in which the cells develop.
- Genetic control of cerebellum development
One of the advantages of evolutionary theory in biology is the possibility of formulating hypotheses in other groups of organisms based on knowledge in a particular group. The cerebellum is a perfect example of the above. Due to the great ease of obtaining mutants in organisms such as Drosophila, many genes involved in segment identity were identified in the second half of the 20th century. Because these genes were capable of establishing anterior-posterior segment identity in Drosophila, several investigators hypothesized that mammalian homologues might control patterns of development. Candidate genes were En (Engrailed), wingless, and Pax genes. Searching for their homologues in vertebrates and analyzing the mutants found a very fine pathway for the control of spatial and temporal development of the cerebellum in mice.
Mutations in the gene (-/-) generate a phenotype that practically does not develop a cerebellum. While mutations in the En-2 gene generate a less severe phenotype, with damage in the formation of leaf structures of the cerebellar lobes. Conditional mutants for En-1 activated on day E-9 whose En-2 expression is normal exhibit nearly normal phenotypes. This suggests that En1 determines the "Territory" of the cerebellum in early stages, whereas En2 is required in later stages. Due to the regulatory effect of Wnt-1 (wingless homologue) and Pax genes on Engrailed, the phenotype of Engrailed was predictable. mutants for these genes. Homozygous Wnt-1 mutants showed complete loss of the cerebellum, which correlates with loss of En expression in the "cerebellar territory".
- Development programmes in the cerebellum
The transition from progenitor cell to mature neuron involves a series of morphological and molecular changes highly regulated spatially and temporally. These changes include the arrest of the mitotic cycle, the formation of axons and dendrites, the expression of specific proteins such as channel proteins, in some cases migrations and finally the establishment of connectivity (synapses) with other neurons. Despite being routines that include most of these processes, different cell types present their programs in different orders. For example, Purkinje cells, like cells of the cerebral cortex, migrate just after exiting the cell cycle and form axonal connections at later stages of development. In contrast, granule cell precursor cells initiate axonal growth upon exiting the cell cycle and subsequently initiate their migration to the inner layer (IGL). Some features of cerebellar granule cell development are shown below.
- Granular cells
The patterns of gene expression during the development of granule cells allow us to establish four stages: neurogenesis, the beginning of neuronal differentiation, axonal growth and migration, and, finally, the formation of synaptic connections. Figure 1 shows the specific markers for each stage.
- Proliferation
The proliferation process occurs mainly in the outer layer of the EGL (oEGL) during the first three postnatal weeks in mice. The first in vitro proliferation studies showed that these cells have the ability to proliferate in the absence of mitogens, suggesting autocrine activity in the regulation of cell proliferation. More recently, some signaling molecules have been shown whose relationship with proliferation is clearer. Wechsler and Scott from Stanford University showed the expression of Shh messengers in Purkinje cells at the somatic and dendritic level, while the granule cells expressed the ptc gene (inhibitor of the shh pathway in the absence of Shh) and the gli1 genes. /2 encoding transcription factors downstream in the Shh signaling cascade. Then they evaluated the role that Shh plays in the proliferation of granule cells, finding that the presence of this factor increases the proliferation of these cells 100 times. This effect was specific for granule cells (no significant increases in glial cell proliferation were seen). To give biological validity to the in vitro results, the researchers inhibited Shh activity by expressing anti-Shh antibodies by hybridoma cells injected into the animals in the early postnatal period. These experiments caused a marked decrease in the thickness of the outer granular layer (EGL), as well as a decrease in the number of cells. This allows us to conclude the causal effect of Shh signaling in the proliferative stage of granule cells. The presence of ptc2 in granule cells is relevant, since granule cells with the active Shh signaling pathway do not enter the cell differentiation stage, even ptc mutants generate medulloblastoma in mice and humans. Therefore, Shh activity is essential in the early stages of granule cell development (proliferation), but its inhibition and subsequent regulation is necessary to continue the normal developmental course of these cells. A recent article on the subject, which talks about Shh and ATF5 in the control of granule cell proliferation, can be consulted
- Variance
Continuing the process, the granule neurons must terminate cell proliferation induced by mitogenic agents such as Shh. Sato et al. showed the antagonistic effect of JASP1 on Shh through the modulation of JNK activity. Activation of this signaling pathway by fibroblast growth factor FGF-2 produces a colocalization of JASP1 and the phosphorylated forms of JNK and ERK on the cell membrane, which will subsequently lead to the inhibition of Shh mitogenic activity allowing it to exit the cell cycle. This is evidenced by a decrease in the population of cells positive for the Ki67 factor (proliferation) and an increase in cells positive for p27-Kip1 (cell cycle repressor) and BrdU.
Another gene involved in the differentiation-migration interface is the weaver gene. Mutants for this gene have normal granule precursor cell (GCP) proliferation, however these cells cannot exit the cell cycle and end up dying. These cells can express some neuronal markers such as N-CAM, L1 and MAP-2, but the expression of late genes such as TAG-1 and astrotactin is knocked out.
- Migration
Immature granule neurons that initiate cell differentiation begin the formation of an axon with the characteristic T-shape (located towards what will be the molecular layer). This stage of development is identifiable by the presence of TAG-1 in the forming axon. At the other extreme, successive and discrete translocations of the nucleus begin; this process of migration from the EGL to the IGL traversing the Purkinje cell layer (PCL) involves the interaction and direct contact between Berman glial cells and granual neurons. In 1988, through immunological techniques and microscopy, Edmonson et al. discovered the membrane protein astrotactin (ASTN1), a 100 kDa glycoprotein whose function is to stabilize the temporal junctions between astroglia and granule neurons. This article shows how the weaver mutants mentioned in the previous section do not express this protein and, at the same time, are incapable of joining Bergman glial cells and initiating migration.
Recent studies carried out by Dr.Hatten's group have shown the non-redundant activity of ASTN2. This protein was discovered from bioinformatic homology analyses. Incredibly (as the same author says), this protein is not exposed to the cell surface like its ASTN1 counterpart, and therefore cannot have a direct role in neuron-glia adhesion. In a first phase of the study, dynamic control was shown in the exocytosis endocytosis of vesicles with ASTN1, this glycoprotein is exocytosed in the distal area of the leader process (cytoplasmic process that defines the direction of migration) where an adhesion point is required to apply the forces driving somatic translocation. Once this movement has occurred, ASTN1 is required at the new migration frontier and the membrane with ASTN1 that is close to the nucleus is endocytosed for its subsequent recycling. ASTN2 physically interacts with ASTN1 and appears to regulate the amount of ASTN1 that is exported to the membrane.
In addition to glial-neuron cell interactions, granule cells must establish a polarity that directs migration and organize the motor components that execute displacement. In this regard, Solecki et al. have worked on the control of cytoskeletal components in the migration process. First, a box of microtubules is assembled around the nucleus, this is coordinated by the centrosome. The discrete movements of the nucleus are preceded by the advance of the centrosome in the direction of the leader process, which is molecularly coordinated by the Par6 complex (studies are currently being carried out on GTPases that interact with the Par6 complex, which may contribute to explaining the polarity in migration). One of the molecular mechanisms directly responsible for movement is actomyosin motor activation.
- Synaptic connections established
Once migration is complete, neurons are located in the inner granular layer, ready for the process that will turn them into functional neurons: synaptic connections. The T-shaped axons of the molecular layer give rise to connections with the dendrites of Purkinje cells, while the mossy fibers form nerve terminals around the somas of granule neurons (synaptic glomeruli). Another change occurs in the granule cell maturation is the expression of the GABA receptor α6 subunit (remember that electrophysiological modulation depends on active channel receptors, such as the GABA receptor) and the expression of the enzyme glutamic acid dehydrogenase (catalyzes the decarboxylation of glutamate to synthesize GABA). Piper et al. have identified a transcription factor that triggers expression of the GABA receptor α6 subunit in these cells, suggesting that these developmental changes are controlled by divergent cascades (the activation of a few transcription factors is responsible for a very different gene expression profile). Figures 2 and 3 show sections of the adult cerebellum, where the granular layer, the purkinje cell layer and the internal granular layer (IGL) can be identified after migration and establishment of synaptic connections.
Phylogenetic evolution
The cerebellum appears in all vertebrates but with different degrees of development: very small in fish, amphibians and birds, it reaches its maximum size in primates, especially in man.
Anatomy
The cerebellum is attached to the posterior wall of the brainstem and is enclosed within an osteofibrous case—the cerebellar or subtentorial cell—formed by an upper and a lower wall. The upper wall is made up of an extension of the dura mater called the tentorium and the lower wall is formed by the cerebellar fossa of the occipital bone covered by the dura mater. Typically, the cerebellum of an adult male weighs about 150 g (grams) and is 10 cm (centimeters) wide, 5 cm high, and 6 cm anterior-posterior. In children, the ratio between the volume of the cerebellum and the brain is 1:20, while in adults it is 1:8.
External description
The cerebellum is made up of two hemispheres separated by a vermis, it has the shape of a truncated cone flattened in a superior-inferior direction in which it can be differentiated by three faces: superior, inferior and anterior.
Upper face
The upper face has the shape of a tissue with two lateral slopes and is in contact with the tentorium cerebellum. In the central part, it presents an elongated elevation in the anterior-posterior direction that is called the superior vermis. On both sides of the superior vermis extend two inclined and almost flat surfaces that constitute the upper faces of the cerebellar hemispheres. The superior face is separated from the inferior face by the circumferential border of the cerebellum. In a superior view, the circumferential border presents two notches: one anterior in relation to the brainstem, and one posterior in relation to the falx cerebellum. The circumferential border of the cerebellum is covered longitudinally by a deep fissure called the prima fissure or primary sulcus.
Bottom Face
The underside rests directly on the dura mater that lines the cerebellar fossae. It shows a wide groove in the midline called the vallecula or median fissure that houses the falx cerebellum and at the bottom of which is the inferior vermis, which is the continuation of the superior one. Lateral to the median fissure are the undersides of the cerebellar hemispheres, which are convex downward. In the most anterior part and on both sides of the inferior vermis, the cerebellar hemispheres present an ovoid prominence called the cerebellar tonsil. These tonsils are closely related to the medulla oblongata.
Front face
The anterior face is closely related to the posterior face of the brainstem and in order to see it it is necessary to section the three pairs of peduncles that join it. It presents a central depression that corresponds to the roof of the fourth ventricle and is delimited by the peduncles on both sides and by the superior and inferior medullary velums. Above this depression is the anterior end of the superior vermis or lingula, and below is the anterior end of the inferior vermis or nodule. On both sides of the nodule, and below the inferior cerebellar peduncles, are prominences called floccules. The nodule and the follicles are connected to each other by the peduncle of the flocculus which, in part, runs over the inferior medullary veil.
Divisions
There are three different ways to divide the cerebellum: morphologically, phylogenetically, and functionally.
Morphological
Classically, a morphological division is performed that is merely descriptive of the surface of the cerebellum, and has no functional or ontogenetic basis or any application in clinical practice.
The surface of the cerebellum is furrowed by many transverse fissures more or less parallel to each other. Among them there are two that stand out for being the deepest and are used to divide it into lobes. One is the prima or primary fissure that runs along the upper face and divides it approximately into two equal halves, and the other is the posterolateral or dorsolateral fissure that is located on the anterior face in a caudal position with respect to the nodule and flocs.
These fissures delineate the three lobes of the cerebellum: the anterior, posterior, and flocculonodular. Each of these lobes includes a portion that is part of the vermis and another that is part of the cerebellar hemispheres. The portion of the vermis that corresponds to each lobe is subdivided into segments that are generally associated with a pair of lobules located in the cerebellar hemispheres. The subdivision within each of the lobes is determined by the existence of other less deep transverse fissures.
The anterior lobe is located in front of the fissure prima and covers part of the anterior face and part of the superior face. It is subdivided into:
- ♪ (I), which is the most anterior portion of the vermis and joins the upper medulla veil.
- Central lobe (II and III), which stands just above the lynguilla and extends to both sides through the wings of the central lobulillo (H II and H III). The fissure that separates it from the lynguilla receives the name of precentral fissure.
- Culmen (IV and V), which is the most cranial portion of the entire vermis and is laterally associated with the previous portion of the quadrangle lobes (H IV and H V). The fissure that separates it from the central lobe is called postcentral.
The posterior lobe is located between the primal and posterolateral fissures and covers part of the upper face and part of the lower face. It is subdivided into:
- Declive (VI), which descends from the culmen backwards and is laterally associated with the simple lobe or inferoposterior portion of the quadrangular lobe (H VI).
- Folium u leaf of the vermis (VII-A), which is a narrow plate of union between the upper semilunar lobes (or anseriforms; H VII-A) left and right.
- ♪ or tube of the vermis (VII-B), which is laterally associated with the lower semilunar lobes (H VII-A) and the gráciles lobes (slims or paramedians; H VII-B), and is located just below the horizontal fissure that separates it from the folium.
- Pyramid of the Vermis (VIII), which stands ahead of the tuber and is associated with the left and right digressive lobes (H VIII-A and B). The fissure that separates it from the tuber is called prepiramidal and the fissure that separates it from the ovula is called postpiramidal or secondary.
- Vulture of the Vermis (IX), which is between the two cerebelous tonsils (H IX) just above the pyramid.
The floculonodular lobe is located in front of the posterolateral fissure and, as its name indicates, it is made up of the nodule (X) -corresponding to the vermis- and the floccules (H X) -corresponding to the vermis. to the hemispheres-, united by the peduncle of the floc.
The term cerebellar body is used to refer to the entire cerebellum, except for the flocculonodular lobe.
The upper vermis is made up of the lingula, the central lobule, the culmen, the slope, and the folium. The inferior vermis is made up of the tuber, the pyramid, the uvula, and the nodule.
Some authors instead of distinguishing three lobes distinguish four: the anterior, the middle, the posterior and the flocculonodular. The difference is that they divide the posterior lobe in two through the prepyramidal fissure, in such a way that the median lobe extends above it and the posterior lobe below.
Phylogenetics
Phylogenetically, the cerebellum can be divided into three parts: archicerebellum, paleocerebellum, and neocerebellum. This division is of great interest because each of the portions has a certain functional and clinical identity.
The archicerebellum. It is the oldest phylogenetically portion and corresponds to the flocculonodular lobe. It arises during phylogenetic development at the same time as the vestibular apparatus of the inner ear. Most of the afferents it receives come from the vestibular nuclei and largely correspond to the vestibular cerebellum. It has a balance function.
The paleocerebellum. It is more modern than the archaeocerebellum and consists of the pyramid, the uvula, the central lobule with wings, the culmen, and the quadrangular lobule. Most of the afferents it receives come from the spinal cord, and it has some correspondence with the spinocerebellum. It has a postural control function.
The neocerebellum. It is the most modern part and is made up of the entire posterior lobe except for the pyramid and the uvula. Most of the input it receives comes from the cerebral cortex through the pons nuclei and is identified with the cerebrocerebellum. It has a function of motor coordination (voluntary movements).
Topographic representation of the body
Just as the somatosensory cortex, motor cortex, basal ganglia, red nuclei, and reticular formation have a topographical representation of different parts of the body, so too does the cerebellar cortex. The trunk and neck as well as the proximal portions of the extremities are located in the region belonging to the vermis. Instead, the facial regions and the distal portions of the extremities are located in the paravermian bands. The lateral portions of the cerebellar hemispheres (cerebrocerebellum), like the flocculonodular lobe (vestibulocerebellum), do not have a topographical representation of the body.
These topographic representations receive afferents from all respective portions of the body and also from corresponding motor areas in the cerebral cortex and brainstem. In turn, they return motor signals to the same respective areas of the motor cortex and also to the appropriate topographical regions of the red nucleus and reticular formation in the brainstem.
Internal structure
In a similar way to the brain, the cerebellum can be divided into gray matter and white matter. The gray matter is arranged on the surface, where it forms the cerebellar cortex, and in the interior, where it forms the deep nuclei. The white matter is located in the internal part, completely enveloping the deep nuclei.
Cerebellar cortex
The cerebellar cortex has a very large surface area, about 500 cm² (square centimeters) thanks to the numerous folds or convolutions (folia cerebelli) that are predominantly transverse, increasing its area by about three times. The abundant grooves and fissures give the surface a characteristic rough appearance.
The cortex is made up of a multitude of histofunctional units known as cerebellar lamellae. In a sagittal cut of a cerebellar gyrus seen under a microscope, it can be seen that it is made up of a multitude of microgyrici. These microconvolutions are the cerebellar lamellae, which are made up of a thin sheet of white matter covered with gray matter.
The peripheral gray matter of the cerebellar lamella is about 1 mm (millimeter) thick. It has a histological structure, homogeneous in all its regions, made up of three layers in which seven fundamental types of neurons can be distinguished. Like the rest of the nervous system, the cerebellar cortex also contains glial cells and blood vessels.
Layers of the cortex
In the cerebellar cortex, from deep to superficial, the following layers can be distinguished: granular cell layer, middle layer or Purkinje cells, and molecular or plexiform layer.
The granular layer is the deepest layer of the cerebellar cortex and is bordered on the inside by the white matter. It owes its name to the fact that a type of small intrinsic neurons called grains or granular cells of the cerebellum predominate in it. Due to the staining characteristics of the nuclei of these cells, the granular layer presents a lymphocytoid (basophilic) appearance, although small eosinophilic acellular spaces called protoplasmic islands can occasionally be seen. It has a variable width of 500 in the convexity to 100 μm (micrometers) in the sulcus, being the thickest layer of the cerebellar cortex.
The Purkinje cell layer is made up of Purkinje cell somata arranged in a monocellular sheet. At low magnifications, it shows a higher cell density in the convexity of the lamella than in the grooves. Some authors do not consider Purkinje cells to form a defined layer and divide the cerebellar cortex into only two layers: granular and molecular.
The molecular layer gets its name because it contains mainly cell processes and few neuronal cell bodies. It has an eosinophilic staining character (it acquires a pinkish color in sections stained with hematoxylin-eosin). Its approximate thickness is about 300 to 400 μm and its surface is covered by the pia mater.
Neuronal types
The neurons of the cerebellar cortex are classified into: principal or projection neurons and intrinsic or interneurons. The main ones are those whose axons leave the cortex to reach the deep cerebellar nuclei or the vestibular nuclei. The intrinsic ones are those that extend their axons exclusively through the cortex. We also have to take into account the extrinsic afferent fibers that reach the cortex, among which the mossy and climbing fibers stand out.
The principal neurons are the Purkinje cells whose arrangement, shape, and size are homogeneous throughout the cerebellar cortex. It has been estimated that there are about 30 million of these neurons in the human cerebellum. Its soma has a diameter between 40 and 80 μm (micrometers). A thick dendritic trunk originates from the upper part of the neuronal body that branches profusely into first, second, and third order branches, in such a way that they constitute a dense dendritic tree characteristic of these neurons. This dendritic tree extends throughout the entire thickness of the molecular layer, with the particularity that it arborizes practically in a single plane, perpendicular to the transversal axis of the lamella. In this way, in parasagittal sections, the ramifications of these neurons can be seen in all their extension, while in cross sections, their arborization is observed as a few narrow vertical branches. The dendrites are covered with spines, such that it has been estimated that each Purkinje cell may have 30,000 to 60,000 spines. From the lower part of the soma originates the axon which, near its origin, becomes myelinated, passes through the granule cell layer and, after emitting collaterals, enters the white matter. From here the Purkinje cell axons travel to the cerebellar and vestibular nuclei where they terminate. The axonal recurrents return to the Purkinje cell layer in the vicinity of which they arborize, forming the supraganglionic and infraganglionic plexuses. Ultrastructurally, Purkinje cells are characterized in that their soma shows abundant rough endoplasmic reticulum and a highly developed Golgi apparatus. Flattened membranous cisterns belonging to the smooth endoplasmic reticulum just below the membranes (hypolemnal cisterns) frequently appear in the soma, dendrites, and axon. These hypolemnal cisterns are characteristic of this cell type, although some of them can be found in other types of large neurons.
The intrinsic neurons are distributed in the granular and molecular layers. Three types of cells are found in the granular layer: granular cells, large stellate cells—Golgi and Lugaro cells—and monodendritic or monopolar tufted cells. In the molecular layer are the small stellate cells—stellate cells and basket cells.
The granule cells or grains of the cerebellum are the smallest neurons in the entire human nervous system and their soma measures 5 to 8 μm in diameter. They are densely packed in the granular layer. They are very numerous, and it is estimated that there are about 50,000 million of these neurons in the human cerebellum. The soma has hardly any Nissl clumps and is almost completely occupied by the nucleus, which presents dense chromatin, which causes great chromophilicity and is responsible for the lymphocytoid appearance of the cell. The neuronal bodies are not covered by glia and are located very close to each other but without presenting synapses. Four to six short dendrites depart from the soma, about 30 μm in length, with a somewhat flexuous path and without ramifications, which have neurotubules and neurofilaments inside. These dendrites end in several dilations reminiscent of the fingers of a hand, which converge in the protoplasmic islets and through which it establishes synapses with the mossy fibers. From the soma, or from one of its dendrites, the axon starts, unmyelinated throughout its length, which ascends through the molecular layer following a slightly curved path. Once it reaches the surface of the molecular layer, the axon branches into a T, giving rise to two fibers called parallel fibers. These parallel fibers take a transversal path, that is, parallel to the axis of the lamella and perpendicular to the dendritic arborization of the Purkinje cells. The parallel fibers can measure 2 to 3 mm (millimeters) in length, which is extraordinary for a neuron with such a small soma. Normally, the deepest grains have the thickest axons and give rise to the deepest parallel fibers. Through the parallel fibers, the granule cells make synapses en passant with the dendritic spines of the Purkinje cells, so that a single granule cell can contact a variable number (50 to 100) of cells. of Purkinje and, in turn, each of these receives impulses from about 200,000 to 300,000 parallel fibers. This arrangement is reminiscent of that of the poles and cables of a power line. In addition, the parallel fibers also make synapses "en passant" on the dendrites of Golgi cells, basket cells, and stellate cells. Granule cells receive their inputs from mossy fiber rosettes and Golgi cell axons. Both types of terminals synapse on the finger-like varicosities of the granule cells, together forming what is called the cerebellar glomerulus.
The name large stellate cells includes all those neurons, other than grains and tufted monodendritic cells, which are located in the granular layer.
The Golgi cells are somewhat smaller than the Purkinje cells and their number is similar to that of the latter neurons. Its soma is star-shaped and is preferably located in the superficial zone of the granular cell layer. It contains abundant Nissl clumps and neurofibrils, and a smooth endoplasmic reticulum and Golgi apparatus nearly as rich as the Purkinje cell; on the other hand, hypolemnal cisterns are very rare. It presents a notched nucleus, with loose chromatin and a prominent eccentric nucleolus. Its dendrites, four or five in number, start in a horizontal or downward direction, bend and become dichotomized, adopting as a whole the shape of a not very dense bouquet, which projects towards the molecular layer. Dendritic spines are not very abundant. As we move away from the soma, the organelle content of the dendrites decreases and in the most distal regions there are only bundles of neurotubules and some smooth endoplasmic reticulum. Unlike the Purkinje cell, the Golgi cell's dendritic field is arranged in all three dimensions and encompasses a wide territory encompassing an area of about 20 Purkinje cells. From the basal region of the cell or from one of the main dendritic stems an axon in the form of an extraordinarily dense branching plexus lies in the granule cell layer. The axonal plexus of Golgi cells presents three basic types of arborization with a perfect functional correspondence. In the first type, the axonic plexus would cover a field similar to the dendritic field; in the second type, the axon would extend much further but without leaving the lamella; in the third type, two plexuses originate, one in the lamella itself and the other in the neighboring one. The axon plexus terminates in numerous clustered groups of endings that coalesce at protoplasmic islets and synapse on granule cell dendrites. Golgi cells receive their input from mossy fibers and climbing fibers and, to a lesser extent, from other neurons such as granule cells. A characteristic type of synapse is the axo-somatic synapse formed by a dilatation of mossy fibers that embeds itself in the body of a Golgi cell, being almost surrounded by its cytoplasm.
The Lugaro cells are not as well known or studied as other neuronal types of the cerebellum. They are characterized by having a large spindle-shaped soma located just below the Purkinje cell layer. They have long rectilinear or fan-shaped oppopolar dendrites, which extend in a transverse plane and cover a field containing 1-2 complete rows of Purkinje cells. Its axon bifurcates into a broad beaded plexus that extends from the top of the granular layer to the surface of the molecular layer, arranged in a sagittal plane.
Apart from Golgi and Lugaro cells, there are other types of cells that are also large stellate cells. These are aberrant elements and, therefore, very infrequent and with little functional significance. They are Golgi cells, Purkinje cells, and projection neurons from the deep nuclei, in an ectopic situation.
The tufted monodendritic cells are a new cell type recently described. They are found in the granular layer, have a spherical soma and a single dendritic trunk that ends in a short tufted arborization.
The small stellate cells can be superficial (stellate cells) or deep (basket cells).
basket cells are a special type of small stellate cells that Cajal called «small deep stellate cells». In the human cerebellum, there are about 90 million basket cells. They are characterized by the fact that their soma is triangular or stellate in shape with a diameter of 10 to 20 μm and is located in the inner half of the molecular layer just above the Purkinje cells. It has a lobed and eccentric nucleus, and its cytoplasm has a few organelles concentrated at the opposite pole to the nucleus. Nissl lumps and hypolemnal cisterns are rare, and the Golgi apparatus and smooth endoplasmic reticulum are underdeveloped. Their dendrites may be descending, although they usually ascend to the upper third of the molecular layer, are between 100 and 200 μm long, and are oriented in approximately the same plane as Purkinje cells. The dendrites are rectilinear, almost without ramifications and with spines, although much less abundant and coarser than those of Purkinje cells. They have abundant neurotubules, neurofilaments, and smooth endoplasmic reticulum even in their most distal portions, and mitochondria, rough endoplasmic reticulum, and Golgi apparatus in the main dendritic trunks. The axon, which can reach 1 mm (millimeter) in length, after traversing a horizontal path in the sagittal plane, increases in caliber, emits collaterals to the molecular layer, and ends in a series of terminals that surround the Purkinje cell somas. establishing numerous synaptic contacts. These axon terminals form a kind of basket -for which these neurons receive their characteristic name- converging at the base of the Purkinje cell soma where they form a brush that surrounds the initial segment of the axon. Each axon of a basket cell can give rise to about ten perisomatic baskets, while several basket cells contribute to the pericellular nests of a Purkinje cell. In contrast to other neurons in the cerebellum, the axonal fields of basket cells show marked overlap. Basket cell inputs come mainly from climbing and parallel fibers, as well as stellate cells, collaterals from the supraganglionic plexus of Purkinje cells, and other basket cells.
Within stellate cells several different types can be distinguished, although their general morphology is essentially similar in all of them. Its soma is stellate or polygonal and is located in the external part of the molecular layer. It has a nucleus with loose chromatin and a cytoplasm with few organelles. Its axon, after an initial stretch of 5 to 6 μm in length, branches off near the soma, forming a plexus that ends up making synapses on different areas of the Purkinje cell and on other interneurons. Its dendrites originate from five or six main trunks and branch in the transverse plane forming a spiny varicose plexus that extends through the molecular layer receiving synapses from parallel and climbing fibers as well as other stellate cells and basket cells. In addition, there are other stellate cells that are somewhat larger and have an appearance very similar to that of basket cells, even participating in the formation of perisomatic baskets, although without being part of the brush.
Extrinsic fibers
Extrinsic fibers are afferent myelinated axons that reach the cerebellar cortex from other regions of the central nervous system. The most important are the mossy and climbing fibers.
The mossy fibers are thick myelinated fibers that come from numerous areas of the nervous system such as the vestibular ganglion and nuclei, the spinal cord, the reticular formation, and the pons nuclei. Through these fibers, the cerebellum receives information from practically the entire nervous system, including the cerebral cortex. They enter mainly through the middle and superior cerebellar peduncles, and provide collaterals for the deep nuclei, subsequently distributing throughout the cerebellar cortex. Upon reaching the granular layer, the mossy fibers follow a tortuous path and divide into several branches that present arborized and varicose dilations similar to moss and called rosettes or rosaceous. Each mossy fiber gives rise to about 20 rosettes that are located both in the course of the fiber and in its terminations and bifurcations. These rosettes synapse on the fingerlike dilations of the granule cells and the axons of the Golgi cells, forming the so-called cerebellar glomeruli. They also synapse with the soma of the Golgi cells.
Mossy fibers are thick, with abundant neurotubules, neurofilaments, and mitochondria. They are wrapped in a thick myelin sheath in whose Ranvier nodes the rosettes are located.
The climbing fibers are the axons of neurons projecting from the inferior olivary nucleus from where they enter the cerebellum via the inferior peduncle. A single neuron in the inferior olivary nucleus gives rise to about ten climbing fibers. They have smaller diameter than the mosses. Upon reaching the cerebellum, these fibers provide collaterals for the deep nuclei and then distribute throughout the cerebellar cortex where they lose their myelin. They penetrate the granular layer in a straight line and without varicosities, giving one or two collaterals. Reaching the Purkinje cell layer where each fiber overlaps several Purkinje cells ascending over them while branching. There is one climbing fiber for every 5 to 10 Purkinje cells that makes about 300 synapses with each neuron. The fate of the collaterals of the granular layer are the dendrites and somata of the Golgi cells.
Climbing fibers in their most distal portion become thin and unmyelinated, with few neurofilaments, few mitochondria, and abundant "en passant" with the dendrites of Purkinje cells. They also have very dense buttons full of rounded vesicles that demonstrate the existence of synapses between these fibers and the dendrites of stellate cells and basket cells.
In addition to the mossy and climbing fibers, the cerebellar cortex receives other afferent nerve fibers, including those from the locus caeruleus, which are noradrenergic and are distributed throughout the three layers, and those originating from the raphe nuclei, which send serotonin to the granule cell layer and the molecular layer.
Glia
The cerebellar cortex is dominated by protoplasmic astrocytes, including a peculiar type of astrocyte called Bergmann glia. The soma of this cell is irregularly shaped and is found between the Purkinje cells from which two to three processes with thick protoplasmic outgrowths extend through the entire molecular layer and reach the pia mater. Once the pia mater is reached, they are attached to it by means of enlargements that form the limiting layer of Cajal. Another special type of astrocytes are the Fañanás cells whose cell bodies are located in the molecular layer and their expansions do not reach the pia mater. Both the Fañanás cells and the Bergmann glia did not present any ultrastructural peculiarities, both expressing positivity for the gliofibrillary acidic protein (GFAP) antibody.
In the granular layer, protoplasmic astrocytes can be observed that do not isolate all the neurons and that seem to form circles around the cerebellar glomeruli. Likewise, there are oligodendrocytes in the molecular layer but not in the granular one.
Deep Cores
Inside the white matter we can find 4 pairs of gray matter nuclei, which from medial to lateral are: the fastigial (or roof) nucleus, the globose, the emboliform and the dentate. The emboliform and the globose are closely related functionally and together form the interposed nucleus. The vestibular nuclei of the medulla oblongata also function in some respects as though they were deep cerebellar nuclei because of their direct connections to the cortex of the flocculonodular lobe.
The nucleus of the fastigium is a thick comet-shaped mass, located nearly midline, just above the roof of the fourth ventricle from which it is separated by a thin layer of white matter. The globose nucleus is elongated anteroposteriorly and lies between the fastigial and emboliform nuclei. The emboliform nucleus is comma-shaped, with the thick part directed anteriorly, and lies next to the hilum of the dentate nucleus.
The dentate nucleus is the largest and has been estimated to contain about 250,000 neurons. It is yellowish-gray in color and has the shape of a folded pouch open forward and toward the midline. The opening is called the hilum of the dentate nucleus, and through it exit most of the fibers that form the superior cerebellar peduncle. In the dentate nucleus, at least two types of neurons are distinguished: the large or projection neurons and the small ones or interneurons. But the synaptic circuitry of this nucleus is not clearly established. Both the projection neurons and the interneurons have not very numerous, long and slightly branched processes, which give them a general star-shaped appearance.
The dentate nucleus, like the rest of the cerebellar nuclei, in addition to receiving collaterals from fibers that reach the cerebellum from other nerve centers, receives the axons of the Purkinje cells. Each of these axons terminates in a dilated terminal plexus over about 30 neurons in the cerebellar nuclei. The axons of the projection neurons are directed through the peduncles towards specific nerve centers. There are no direct connections from the cerebellar cortex to the outside, except for a few Purkinje cell axons that directly reach the vestibular nuclei.
White matter
In a sagittal section of the cerebellum, the white matter adopts an arborescent arrangement, which is why it is sometimes known as the cerebellar tree of life or arbor vitae. It is formed by a voluminous central mass, called the body or medullary center, from which extensions towards the gyri of the cerebellum called white plates start. The medullary body is continued forward directly with the peduncles, which are also made of white matter.
Histologically, the white matter of the cerebellum is made up of axons together with fibrous astrocytes and abundant oligodendrocytes that produce the myelin sheath. White matter axons are both efferent and afferent fibers as well as intrinsic fibers that connect different cortical areas with each other. The afferent fibers of the cortex correspond to axons of the Purkinje cells while those of the deep nuclei correspond to axons of the projection neurons of said nuclei. The afferents correspond to the mossy fibers, the climbing ones and those that come from the noradrenergic and serotonergic systems. Among the intrinsic or own fibers, two types are distinguished: commissural fibers and arcuate or association fibers. The commissurals cross the midline and connect the opposite halves of the cerebellum while the arcades connect adjacent cerebellar gyri to each other.
Gray Matter
The gray matter (or gray matter) corresponds to those grayish areas of the central nervous system made up mainly of neuronal cell bodies and dendrites lacking myelin along with glial cells (neuroglia). In the spinal cord, it can be seen in its center and to the sides, in the shape of a butterfly or letter H, while in the brain it occupies the external zone, with the exception of the internal basal ganglia that serve as relay stations. In the brain, it is arranged on its surface and forms the cerebral cortex, which corresponds to the most complex organization of the entire nervous system. This has some functions of regulating, controlling and modulating the operation of the motor impulse.
Cerebellar Connections
The cerebellum receives afferents from all the motor and sensory pathways, including the olfactory one, and efferents depart from it to control all the descending motor pathways. Outputs do not usually synapse directly on motor neurons of the common final pathway except on those of the extrinsic muscles of the eyeball. Efferents normally act on the motor nuclei of the brainstem. The number of cerebellar afferent fibers is more than 40 times greater than that of efferent fibers. All connections to the cerebellum pass through the peduncles.
Next, the main connections established by the cerebellum will be explained, ordered according to its functional division. It must be taken into account that the afferent fibers, unlike the efferent ones, do not terminate on the cerebellar cortex strictly following the functional division.
Afferents from the vestibulocerebellum
Mostly they come from the vestibular system through two tracts: the direct or Edinger vestibulocerebellar and the indirect vestibular cerebellar. It also receives some fibers from the cerebellar corticopontic tract that come from the visual cortex of the occipital lobe (occipitoponticocerebellar fibers).
The direct vestibulocerebellar or Edinger tract is formed by the axons of neurons located in the vestibular or Scarpa ganglion, which preferentially reach the node and some reach the vermian band. It bypasses the vestibular nuclei, does not decussate on its way, and enters directly through the inferior peduncle. It transmits information about the position of the head and the linear and angular accelerations that the body undergoes.
The indirect vestibulocerebellar tract is formed by the axons of the neurons located in the superior and medial vestibular nuclei, which end in the floccules and, to a lesser extent, in the vermian band. It does not decussate on its way and enters through the inferior peduncle. It transmits information about the position of the head and the linear and angular accelerations that the body undergoes.
Efferences from the vestibule-cerebellum
The main fiber tracts that originate from the vestibular cerebellum are: the cerebellovestibular, the flocculoculomotor, and the uncinate of Russell.
The cerebellovestibular tract is made up of direct and crossed fibers originating from the flocculi and leaving the cerebellum via the inferior peduncle to reach the medial and lateral vestibular nuclei. It regulates the activity of the medial and lateral vestibulospinal tracts.
The flocculoculomotor tract originates in the floccules, decussates in the middle of the cerebellum, exits through the superior peduncle, and ascends through the brainstem until it reaches the nucleus of the oculomotor (or oculomotor) nerve.. Controls the movements of the eyeball.
The Russell's uncinate tract originates from the floccules, crosses, and passes cranially toward the superior cerebellar peduncle. But before reaching that peduncle, it abruptly changes direction, forming a kind of hook and ends up coming out from the bottom. It ends in the vestibular nuclei. During its course in the cerebellum, it emits collaterals that exit through the superior peduncle and reach the nuclei of the oculomotor nerves, the reticular formation, and the hypothalamus. It controls the movements of the eyeball and the activity of the vestibulospinal tracts.
Spinocerebellar Inputs
Input to the spinocerebellum comes from three areas of the neuraxis: the spinal cord, the medulla oblongata, and the midbrain.
At the level of the spinal cord, inputs arrive via the posterior and anterior spinocerebellar tracts. These tracts are capable of transmitting nerve impulses faster than any other pathway in the CNS, reaching a speed of 120 m/s (meters per second). This speed is necessary for the information about the changes that have occurred in the peripheral muscle groups to reach the cerebellum and to be able to coordinate them in time.
The anterior (ventral) or Gowers spinocerebellar tract originates in the medulla, in neurons that sit lateral to the base of the dorsal horn, between the last lumbar segments and the sacrococcygeal segments. Some of its fibers cross the gray commissure to ascend the lateral cord on the opposite side, where it lies close to the medullary surface. The few fibers that do not cross ascend the lateral cord on the same side. All its fibers cross the medulla and the pons, and reach the most caudal area of the midbrain where they abruptly change direction to enter the cerebellum through the superior peduncle. It reaches the vermis and the paravermian bands on both sides. It transmits unconscious proprioceptive and exterioceptive information from the lower extremity.
The posterior spinocerebellar tract (dorsal) or of Flechsing is formed by axons of neurons whose soma is located in the thoracic spine or Stilling-Clarke nucleus. It ascends along the lateral cord attached to the surface and just posterior to the anterior spinocerebellar tract. Upon reaching the bulb, it enters the cerebellum through the inferior peduncle and reaches the vermis and the paravermian band on the same side of its origin. It transmits unconscious proprioceptive and exteroceptive information from the trunk and lower extremity.
At the level of the medulla oblongata, inputs arrive via the cuneocerebellar, olivocerebellar, and reticulocerebellar tracts.
The cuneocerebellar tract is formed by the axons of the neurons that sit in the accessory cuneiform nucleus (posterior external arcuate fibers). It ascends through the medulla oblongata without decussation and blends with the posterior spinocerebellar tract. It enters through the inferior cerebellar peduncle and ends in the vermis and the paravermian band on the same side. It transmits the unconscious proprioceptive and exteroceptive sensitivity of the upper half of the body.
The olivocerebellar tract is the most important connection between the medulla oblongata and the cerebellum. It is made up of axons from neurons of the inferior olivary nucleus and the accessory olivary nuclei. These nuclei receive somatoesthetic, visual, and cerebral cortex information, in addition to receiving vestibular afferents and from the cerebellum itself. Shortly after originating, the olivocerebellar tract decussates completely and enters the cerebellum via the inferior peduncle. It ends up providing climbing fibers for the entire cerebellar cortex. It transmits to the cerebellum the information received by the olivary nuclei.
The reticulocerebellar tract is formed by axons of neurons located in the medullary and pontic reticular formation. Part of the fibers cross and another part go direct. It enters through the inferior cerebellar peduncle and reaches mainly the spinocerebellum, although it also sends some fibers to the cerebrocerebellum. It transmits complex information, both from the periphery and from the cerebral cortex and other parts of the central nervous system.
At the level of the midbrain, inputs arrive via the tectocerebellar, trigeminocerebellar, and rubrocerebellar tracts.
The tectocerebellar tract is formed by the axons of the neurons of the superior and inferior quadrigeminal tubercles. They enter the cerebellum through the superior peduncle on the same side and terminate in the middle part of the vermis. It transmits visual and acoustic information from the cerebral cortex.
The trigeminocerebellar tract is formed by axons of neurons from the mesencephalic nucleus of the trigeminal nerve that enter the cerebellum through the superior peduncle without decussation along the way. They terminate in the vermis and in the vermian band on the same side as their origin. It transmits proprioceptive information from the craniofacial massif.
The rubrocerebellar tract is made up of axons of neurons located in the parvocellular portion of the red nucleus that fully decussate before reaching the cerebellum via the superior peduncle.
Spinocerebellar inputs
The main references that start from the spinocerebellum are: the interpuestoreticular tract, the interpuestorolivary tract, the interpuestotectal tract, and the interpuestorrubric tract.
The interpuestoreticular tract originates from the interposed nucleus, its fibers partially decussate and exit the cerebellum via the inferior peduncles to reach the nuclei of the reticular formation.
The interpuestolivar tract exits through the superior cerebellar peduncle, decussates entirely at the level of the midbrain, and descends through the brainstem to reach the inferior olivary nucleus.
The interpuestotectal tract partially decussates before exiting the superior cerebellar peduncle and ascending the brainstem to reach the superior and inferior quadrigeminal tubercles.
The interpuestorubric tract is the most important output of the spinocerebellum and the main route of discharge from the interposed nucleus. The fibers that make it up leave the cerebellum through the superior peduncle, decussate entirely in the midbrain and reach the contralateral red nucleus. From the red nucleus, axons depart to the ventral intermediate nucleus of the thalamus, which, in turn, sends axons to the motor and sensory cerebral cortex. It controls the activity of the motor pathways that descend to the spinal cord.
Cerebrocerebellar afferents
All inputs received by the cerebrocerebellum are part of the corticoponticocerebellar tract. This tract originates in a large area of the cerebral cortex that encompasses the frontal, parietal, occipital, and temporal lobes, and synapses in the pons nuclei before entering the cerebellum.
Most of the fibers that go from the cortex to the pons nuclei are collaterals of axons that go to other areas of the brain or to the spinal cord and whose neuronal body is located in layer V of the cerebral cortex. These fibers can be divided, according to their origin, into: frontopontic, parietopontic, occipitopontic and temporopontic.
The frontopontic fibers originate from the motor and premotor cortices and pass through the anterior arm of the internal capsule. In the midbrain, they course along the base of the cerebral peduncles medially to the corticonuclear tract. They terminate in the more medial pons nuclei.
The parietopontic fibers originate from the primary and secondary somatosensory areas and from visual areas. They pass through the posterior arm of the internal capsule and then through the base of the cereberal peduncles laterally to the corticospinal tract. They terminate in the lateral pons nuclei.
The occipitopontic fibers originate in secondary areas related to the processing of visual stimuli of movement (magnocellular current of the optic pathway). They pass through the retrolenticular portion of the internal capsule and then through the base of the cereberal peduncles laterally to the corticospinal tract. They terminate in the lateral pons nuclei.
The temporopontic fibers pass through the sublenticular portion of the internal capsule and, at the level of the midbrain, are positioned laterally to the corticospinal tract. Terminates in the lateral pons nuclei.
Fibers from the pontine nuclei to the cerebellum (pontocerebellar fibers) course horizontally through the pons, decussate, and enter via the median peduncle. They terminate in the cortex of the hemispheres and in the globose nucleus.
Cerebrocerebellar inputs
Most output from the cerebrocerebellum exits through the dentatothalamic tract. This tract is formed by the axons of neurons located in the dentate nucleus, which exit the cerebellum through the superior peduncle. They decussate in the caudal portion of the midbrain (Wernekink's decussation) and terminate in the ventral intermediate nucleus of the thalamus. From the thalamus originate thalamocortical fibers that reach the same areas of the cerebral cortex from which the corticoponticocerebellar afferents originated.
There is a group of fibers called dentorubrics, which, starting from the dentate nucleus, exit through the superior cerebellar peduncle, decussate and reach the contralateral red nucleus.
Inputs from monoaminergic systems
The cerebellum, like other parts of the CNS, receives fibers from modulatory neurochemical systems. Specifically of two of the monoaminergic systems: the noradrenergic and the serotonergic.
The noradrenergic system sends the caeruleocerebellar tract from group A6 (which coincides with the locus caeruleus) to the cerebellum. This tract penetrates through the superior peduncle and ends up distributed throughout the nuclei and cortex. Its fibers do not behave like mossy fibers or climbers, but rather as diffuse projections.
The cerebellar serotonergic tract originates in groups B5 and B6, enters through the median peduncle and ends up distributed throughout all the nuclei and cortex. Its fibers end in diffuse projections.
Peduncles
The cerebellum is attached to the posterior aspect of the brainstem by 3 pairs of peduncles through which all the nerve fibers that enter and leave it run. There are two lower peduncles, two middle peduncles, and two upper peduncles.
Inferior cerebellar peduncles
The inferior cerebellar peduncles or restiform bodies connect the cerebellum to the upper part of the medulla oblongata. Between them extends the inferior medullary veil. Through them enter the fibers of the dorsal spinocerebellar tract, those of the cuneocerebellar tract, those of the vestibulocerebellar tracts, those of the reticulocerebellar tract, and the climbing fibers from the inferior olivary nucleus and accessories (olivocerebellar tract). Through them exit the fibers of the cerebellovestibular tract, those of Russell's uncinate tract, and those of the interpuestoreticular tract.
Medium cerebellar peduncles
The pontine or middle cerebellar peduncles connect the cerebellum to the pons. They are the largest and are separated from the upper peduncles by the interpeduncular groove. They constitute the lateral faces of the bulge. Through them enter the fibers of the corticopontocerebellar tract and those of the cerebellar serotonergic tract. No major efferent fibers exit through them.
The fibers of the median peduncles are organized into three fascicles: superior, inferior, and deep.
The superior fasciculus, the most superficial, derives from the superior transverse fibers of the pons. It is directed dorsally and laterally, superficially crossing the other two fascicles. It is distributed mainly by the lobules of the inferior face of the cerebellar hemispheres and by the adjacent portions of the superior face.
The inferior fasciculus is made up of the inferior transverse fibers of the pons. It passes deeply into the superior fasciculus and continues backwards and downwards more or less parallel to it. It is distributed by the lobules of the inferior face in the portions close to the vermis.
The deep fasciculus includes most of the deep transverse fibers of the pons. In its first sections it is covered by the inferior and superior fascicles, but it ends up crossing obliquely and appears on the medial side of the superior fascicle, from which it receives a bundle of fibers. Its fibers disintegrate and end in the lobules of the anterior part of the upper face. The fibers of this fascicle cover those of the restiform body.
Superior cerebellar peduncles
The superior cerebellar peduncles connect the cerebellum to the midbrain. Between these two peduncles the superior medullary veil extends. Through them enter the fibers of the ventral spinocerebellar tract, those of the tectocerebellar tract, those of the trigeminocerebellar tract, those of the rubrocerebellar tract, and those of the caeruleocerebellar tract. Through them exit the fibers of the flocculoculomotor tract, those of the interpuestolivar, those of the interpuestorubric, those of the interpuestotectal, those of the dentatothalamic tract, the dentatorubrics, and the collaterals of Russell's uncinate.
Arterial supply
There are three main pairs of arteries that supply the cerebellum: the superior cerebellar arteries (SCA), the inferior anterior cerebellar arteries (AICA), and the posterior inferior cerebellar arteries (PICA).
Superior cerebellar artery
It arises from the basilar artery just below where it divides into its two terminal branches. It goes laterally and backwards, surrounding the corresponding cerebellar peduncle, at the level of the pontomesencephalic groove. It passes immediately below the oculomotor nerve (III) and crosses the cistern ambients accompanying the trochlear nerve (IV). Its terminal branches run through the pia mater, between the tentorium and the superior surface of the cerebellum. It anastomoses with the inferior cerebellar arteries. It supplies the superior cerebellar cortex and deep nuclei, as well as the superior and middle cerebellar peduncles.
As it encircles the midbrain, the superior cerebellar artery gives off the rhomboid artery which follows the superior cerebellar peduncle and enters the interior of the cerebellum to supply the deep nuclei. It also gives off several collateral branches that reach the pineal gland, the superior medullary veil, and the choroidal tissue of the third ventricle.
Anterior inferior cerebellar artery
It arises from the basilar artery just above where it is formed by the union of the two vertebral arteries. It runs laterally and posteriorly, contouring the lateral aspect of the pons just below the apparent origin of the trigeminal nerve (V). It continues its course along the lower border of the middle cerebellar peduncle. It supplies the anterior portion of the underside of the cerebellum, as well as the facial (VII) and vestibulocochlear (VIII) nerves. Its terminal branches anastomose with those of the inferior posterior and superior cerebellar arteries.
In some people, the inferior cerebellar artery gives off the internal auditory or labyrinthine artery (in other people the labyrinthine artery originates from the basilar artery). This branch accompanies the vestibulocochlear nerve (VIII) through the internal auditory canal to reach the middle ear.
Posterior inferior cerebellar artery
It arises from the vertebral arteries just below where they join to form the basilar artery. It runs backwards around the upper part of the medulla oblongata and passes between the origin of the vagus nerve (X) and the accessory nerve (XI). It follows its course on the inferior cerebellar peduncle and when it reaches the underside of the cerebellum it divides into two terminal branches: one medial and one lateral. The medial branch is continued posteriorly through the median fissure, between the two cerebellar hemispheres. The lateral branch runs along the inferior surface of the hemispheres until it reaches the circumferential border, where it anastomoses with the inferior anterior and superior cerebellar arteries.
Supplies the posterior undersurface of the cerebellum, the inferior cerebellar peduncle, the nucleus ambiguus, the motor nucleus of the vagus nerve, the spinal nucleus of the trigeminal nerve, the solitary nucleus, the vestibular nuclei, and the cochlear nuclei.
Its most important collateral branches are the choroidal branch of the fourth ventricle and the medial and lateral bulbar branches. The first contributes to the choroid plexus of the fourth ventricle, and the other two supply the medulla oblongata and the inferior cerebellar peduncle.
Venous drainage
The main veins that drain blood from the cerebellum are: the superior cerebellar veins, the superior vermis vein, the precentral cerebellar vein, the inferior cerebellar veins, the inferior vermis vein, and the petrous veins. All of them end up sending blood to the venous sinuses of the dura mater.
The superior cerebellar veins collect blood from the lateral portion of the superior aspect of the cerebellar hemispheres and normally empty into the transverse sinus.
The superior vein of the vermis collects blood from the superior vermis and empties into the straight sinus through the internal cerebral vein or the great cerebral vein (vein of Galen).
The precentral vein of the cerebellum collects blood from the lingula and central lobule, and empties into the great cerebral vein.
The inferior cerebellar veins collect blood from the lateral portion of the underside of the cerebellar hemispheres and empty into the transverse, occipital, and superior petrosal sinuses.
The inferior vermis vein collects blood from the inferior vermis and empties directly into the straight sinus.
The petrous veins collect blood from the flocculus region and empty into the inferior or superior petrosal sinus.
- Systematization of the faces of the cerebellum
- upper occipital lobe
- previous cerebral stem
- back internal occipital protuberance lateral edges
- lower cerebelous pit
- thorn-talamic-dorsal lingula via self-absorbing of pain arms and legs
Neural Circuits
Taken together, the neural connections in the cerebellum can be divided into: afferent axons, which transmit information from other parts of the CNS to the cerebellum; intrinsic cerebellar circuits—cortical and nuclear—that integrate and process information; and efferent axons, which transmit processed information to other parts of the CNS.
The axons or afferent fibers reach the cerebellar cortex after giving collaterals to the deep cerebellar nuclei or to the vestibular nuclei. In turn, the information is processed in the intrinsic circuits of the cerebellar cortex, and the result, in the form of nerve impulses, is sent by the axons of the Purkinje cells to the deep nuclei. Information is also processed in these nuclei and the efferent fibers of the cerebellum depart from them both in an ascending direction, towards the thalamus and cortex, and descending, towards the spinal cord.
In this way, the basic functional circuit of the cerebellum is made up of two arches: one main or excitatory, which passes through the deep nuclei, and another secondary or inhibitory, which passes through the cortex and regulates the previous one. This circuit is repeated some 30 million times throughout the cerebellum and consists of a single Purkinje cell and the corresponding projection nuclear neuron plus related interneurons.
The basic functional circuit and the cellular elements that make it up are identical in all parts of the cerebellum, for this reason it is considered that information is processed in a similar way throughout the cerebellum.
Neural circuits of the deep nuclei: main arch
The main arch is made up of the collateral branches of the mossy and climbing fibers, which end in the neurons of the deep nuclei. The axons of projection neurons from the deep nuclei exit the cerebellum via the peduncles to terminate in different nuclei in the brainstem and in the thalamus.
In the deep nuclei, mainly axodendritic and some axosomatic synapses are found, although there are also more complex arrangements such as serial and triad synapses. The most frequent synapse is the excitatory axodendritic synapse that is established between a terminal of the axonal collaterals of the mossy or climbing fibers —as a presynaptic element— and a dendrite of a projection neuron or an interneuron of the deep nuclei —a postsynaptic element. The collaterals of the mossy fibers and the climbing fibers use glutamate as their main neurotransmitter, although they can also use other neurotransmitters (especially the mossy fibers). The synaptic circuits that take place between the neurons of the deep nuclei are poorly understood.
From a functional point of view, the deep nuclei of the cerebellum have two basic types of projection neuron: small GABAergic (inhibitory) neurons that send their axon towards the inferior olivary nucleus, and other glutaminergic (excitatory) neurons that They send their axons to other nerve centers.
Deep nuclei projection neurons under normal conditions permanently fire action potentials at a rate of more than 100 per second. This frequency can be modulated up or down depending on the excitatory and inhibitory signals that reach the neuron. Excitatory signals come mainly from the axonal collaterals of the mossy and climbing fibers, whereas inhibitory signals come from the Purkinje cell axons, which are part of the secondary arch. The balance between these two effects is slightly favorable to arousal, which explains why the firing rate of projection neurons remains relatively constant at a moderate level of continuous stimulation.
Neural Circuits of the Cerebellar Cortex: Secondary Arch
The secondary arch passes through the cerebellar cortex and is built around a fundamental neural component: the Purkinje cell. Two types of circuits end in the Purkinje cell: the excitatory or main circuits, which are the ones that stimulate it, and the inhibitory circuits, formed by inhibitory interneurons. Finally, the axons of the Purkinje cells project onto the neurons of the cerebellar and vestibular nuclei, exerting an inhibitory action on them through GABAergic synapses. In this way, the main exciter arc is modulated and regulated.
To all this we must add that the noradrenergic endings that reach the cerebellum release a diffuse neurotransmitter that produces hyperpolarization of the Purkinje cells.
Exciting Circuits
Purkinje cells can be stimulated by two different pathways: by climbing fibers (direct pathway) or by mossy fibers (indirect pathway).
The climbing fibers, ending on the soma and the dendritic tree of the Purkinje cells, produce a direct and highly specific stimulation through Gray type I synapses that use glutamate as a neurotransmitter. By making multiple contacts with each Purkinje cell, a single climbing fiber produces a much more effective excitatory action than mossy fibers.
Mossy fibers do not act directly on Purkinje cells but rather through excitatory interneurons, granule cells. The presence of excitatory interneurons is very rare in the nervous system and is characteristic of the cerebellar cortex. At the level of the cerebellar glomerulus, the mossy fibers form Gray type I (excitatory) synapses on the dendrites of the granule cells and the impulses are carried by the parallel fibers until they reach the dendrites of the Purkinje cells. Parallel fibers present synapses containing spherical glutamate vesicles and type I Gray conformation, consistent with their excitatory character. Taken together, mossy fibers act on Purkinje cells with much convergence and divergence, establishing more nonspecific connections than climbing fibers.
Purkinje cells do not comply with the principle that all action potentials produced by a neuron are the same because they present two different types of action potentials depending on the pathway by which they are stimulated. If stimulated directly through the climbing fibers, they generate prolonged depolarization and a complex spike action potential with a firing frequency of 3 or 4 Hz. When stimulated indirectly through the mossy fibers, they generate a brief action potential called a single spike, with a discharge frequency of 100 to 200 Hz. Generating a single spike requires the temporal and spatial summation of the stimulation produced by several parallel fibers. All this shows that the information provided by the two types of extrinsic fibers that reach the cerebellum is different and is processed in a different way.
Inhibitory Circuits
Inhibitory circuits are made up of the three main types of inhibitory interneurons: Golgi cells, stellate cells, and basket cells. They may act directly on Purkinje cells—as stellate cells and basket cells do—or indirectly through granule cells—as Golgi cells do. All these interneurons use GABA as an inhibitory neurotransmitter.
The stellate cells and the basket cells are stimulated by the parallel fibers of the grains, which have previously been stimulated by the mossy fibers, and are responsible for modulating the activation of the Purkinje cells by the climbing fibers, producing a lateral inhibition phenomenon. This lateral inhibition makes the signal reaching the Purkinje cells more precise in the same way that other mechanisms of lateral inhibition enhance the contrast of signals in many other neural circuits of the nervous system.
Golgi cells receive excitatory stimuli from parallel fibers and, to a lesser extent, from climbing and mossy fibers. They act at the level of the cerebellar glomeruli making type II Gray (inhibitory) synapses on the dendrites of the grains. Through these synpases they modulate granule cell activation by mossy fibers and thus regulate Purkinje cell activity. In this way, the Golgi cells create a negative feedback loop for the granule cells.
Exit Signs
Long-term depression of Purkinje cells: motor learning
Theories on cerebellar function
Modeling of cerebellar function
Pathology
Classically, cerebellar lesions are clinically manifested by:
- Hypotony: It is characterized by a diminished resistance to palpation or passive manipulation of the muscles; it is usually accompanied by decreased and pendular osteotendinous reflections, along with a striking phenomenon of rebounding in the Stewart-Holmes test.
- Ataxia or decoordination of voluntary movements: The alteration of the coordination of the voluntary movements leads to the appearance of hypermetry, assynergia, discronometry and adiadococinsia. In the cerebelous tests (dedo-nariz or heel-rodilla), the speed and start of the movement are not affectionate, but when the finger or heel approach the nose or knee, they exceed their destiny or correct the manoeuvre excessively (hypermetry). Assynergy consists of a breakdown of movement in its constituent parts.
All these disorders are better observed the faster the maneuvers are executed. Adiadochokinesia indicates a difficulty or inability to perform rapid reciprocating movements (puppet test).
- Alteration of balance and march: The alteration of the static causes instability in orthostatism, so the patient must expand its base of support (separate the feet); by standing and walking his body has frequent oscillations. Unlike vestibule disorders, these alterations are not modified by closing your eyes. The march is characteristic and resembles that of a drunkard (bride shell), titubeante, with separate feet and deviating towards the side of the injury.
- Intentional tremor: thick and evident when trying a movement (intentional tremor or action) It must be taken into account that the cerebellum regulates physiological tremor, therefore, its injury causes this type of tremor. Even, there are other types of tremor related directly to the cerebellum: holocraneal tremor or denial, mixed tremor, and dystonic tremor, etc.
- Other: word escandida, explosive, nistagmus, fatigue, etc.
Cerebellar Syndrome
Disease or damage to all or a large part of the cerebellum is known as the cerebellar syndrome. Selective cerebellar lesions are extremely rare.
Cerebellar Vermis Syndrome
The most common cause is medulloblastoma of the vermis in children.
Involvement of the flocculonodular lobe produces signs and symptoms related to the vestibular system. Since the vermis is single and influences midline structures, muscle incoordination affects the head and trunk, not the extremities. There is a tendency to fall forwards or backwards, as well as difficulty keeping the head still and in an erect position.
There may also be difficulty keeping the trunk erect.
Hemispheric cerebellar syndrome
This syndrome may be caused by a tumor or ischemia in a cerebellar hemisphere. In general, the symptoms and signs are unilateral and affect the muscles ipsilateral to the diseased cerebellar hemisphere. The movements of the extremities are altered, especially of the arms and legs, where hypermetria and decomposition of movement are very evident.
Often there is rocking and falling towards the side of the lesion. Dysarthria and nystagmus are also frequent findings.
Etiology of Cerebellar Syndrome
The most frequent etiology of cerebellar syndromes are:
- Vascular:
- Verb-basilar insufficiency
- Infarction
- Hemorrhages
- Trombosis
- Tumors:
- Meduloblastoma (close vein)
- Cystic astrocytoma (cellaneous hemispheries)
- Hemangioblastoma (cellaneous hemispheries)
- Acoustic neurinoma (Photocerebelous clot)
- Metastasis
- Paraneoplasic (lung cancer)
- Traumatics:
- Contusion
- Laceration
- Hematomas
- Toxic:
- Alcohol
- Drugs
- Hidantoinates
- Infectious:
- Virosic cerebelitis
- Supurated cerebelitis
- Abscess
- Tuberculomas
- Degenerative:
- Friedrich's disease
- Pierre-Marie's disease
- Multiple sclerosis
- Autoimmune:
- Gluten ataxia
- Malformations:
- Arnold Chiari
- Dandy Walker's Malformation
- Vascular malformations
Further reading
- Ito M. Cerebellum and Neural Control. New York: Raven Press; 1984. ISBN 0-89004-106-7.
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science4th ed. McGraw-Hill, New York (2000). ISBN 0-8385-7701-6.
- Llinás, R, Sotelo C. The Cerebellum Revisited. New York: Springer; 1992. ISBN 0-387-97693-0.
- Parent A, Carpenter MB. Carpenter's Human Neuroanatomy9th ed. Philadelphia: Williams and Wilkins; 1995. ISBN 0-683-06752-4.
Contenido relacionado
Adenine
Jean Baptiste Lamarck
Pharmaceutical specialty