Cell nucleus
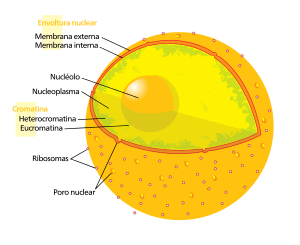
In biology, the cell nucleus is a membranous structure that is normally found in the center of eukaryotic cells. It contains most of the cellular genetic material, organized into several extraordinarily long and linear molecules of DNA, with a wide variety of proteins, such as histones, which make up what we call chromosomes. The set of genes on those chromosomes is called the nuclear genome. The function of the nucleus is to maintain the integrity of these genes and to control cellular activities by regulating gene expression. Therefore, the nucleus is said to be the control center of the cell.
The main structure that makes up the nucleus is the nuclear envelope, a double membrane that completely surrounds the organelle and separates that content from the cytoplasm, in addition to having nuclear pores that allow passage through the membranes for proper regulation of gene expression and chromosome maintenance.
Although the interior of the nucleus does not contain any membranous subcompartments, its contents are to some extent compartmentalized, with a number of subnuclear bodies made up of exclusive types of proteins, different types of RNA molecules, and particular segments of chromosomes, usually divided by the intensity with which they are expressed. The best known of them all is the nucleolus, which is primarily involved in the synthesis of ribosomes. After being produced in the nucleolus, they are exported to the cytoplasm, where, among other things, they translate the mRNA.
History
The nucleus was the first organelle to be discovered. Probably the oldest surviving drawing of this organelle dates back to one of the first microscopists, Anton van Leeuwenhoek (1632–1723). This researcher observed a hole or "lumen", the nucleus, in salmon erythrocytes. Unlike mammalian erythrocytes, those of other vertebrates are nucleated. The nucleus was also described in 1804 by Franz Bauer, and later in more detail by Scottish botanist Robert Brown in a talk given to the Linnean Society of London in 1831. Brown was studying the microscopic structure of orchids when he observed an opaque area, which he called the areola, or nucleus, in the cells of the outer layer of the flower, although he did not suggest a potential function for such a structure. In 1838 Matthias Schleiden proposed that the nucleus played a role in the generation of cells, calling it the "stem cell" (cell builder). He thought that he had observed new cells around these "stem cells". Franz Meyen was a strong opponent of this view, having previously described cells that multiplied by division and believing that many cells would lack a nucleus. He the idea that cells could be generated de novo, either by the & # 34;stem cell & # 34; or otherwise, it contradicted the works of Robert Remak (1852) and Rudolf Virchow (1855) who decisively propagated the new paradigm that cells were only generated by other cells ("Omnis cellula e cellula"). The function of the nucleus remained unclear.
Between 1876 and 1878 Oscar Hertwig published several studies on the fertilization of sea urchin eggs, showing that the nucleus of the spermatozoon entered the oocyte, fusing with its nucleus. This was the first time that it was suggested that an individual developed from a single nucleated cell. This was in contradiction with Ernst Haeckel's theory that the entire phylogeny of a species was repeated during embryonic development, including the generation of the first nucleated cell from a "monerula", a disorganized mass. of primordial mucus ("Urschleim", in German). Therefore, the need for the sperm nucleus for fertilization was under discussion for a while. However, Hertwig confirmed the observation of it in other animal groups, such as amphibians and molluscs. Eduard Strasburger obtained the same results in plants (1884). This paved the way for the assignment of an important role of the nucleus in heredity. In 1873 August Weismann postulated the equivalence of paternal and maternal germ cells in heredity. The function of the nucleus as a carrier of genetic information became apparent only later, after the discovery of mitosis and the rediscovery of Mendelian inheritance at the turn of the century XX. This led to the development of the chromosome theory of heredity.
Structures
The nucleus is the largest organelle in animal cells. In mammalian cells, the average diameter of the nucleus is about 6 micrometers (μm), which occupies about 10% of the total cell volume. In plants, the nucleus is generally between 5 and 25 µm and is visible under a light microscope. In fungi, cases of species with very small nuclei have been observed, around 0.5 µm, which are visible only with an electron microscope. In the oospheres of Cycas and conifers it reaches a size of 0.6 mm, that is, it is visible to the naked eye.
The viscous fluid within it is called the nucleoplasm, and its composition is similar to that found in the cytosol outside the nucleus. It roughly resembles a dense, spherical organelle.
Nuclear envelope and pores
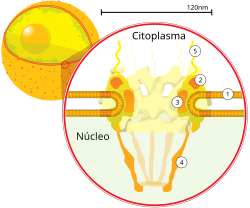
The nuclear envelope is sometimes improperly called the nuclear membrane, because it is composed of, among other things, two membranes, one inner and one outer, arranged in parallel one on top of the other. It is perforated by pores, thanks to these nuclear pores there is a bidirectional movement established between the cytosol of the cell and the nucleus. It prevents macromolecules from diffusing freely between the nucleoplasm and cytoplasm. The outer nuclear membrane is continuous with the rough endoplasmic reticulum (RER) membrane, and is similarly studded with ribosomes. The space between the membranes is known as the perinuclear space or cistern and is continuous with the lumen of the RER.
Nuclear pores, which provide open aqueous channels through which small (ranging from 5,000 to 44,000 Da) and water-soluble molecules can diffuse by passive transport, also prohibit globular proteins larger than 60kDa from entering the nucleus. The size of the channels allows the nuclear compartment and the cytosol to maintain different sets of proteins, they are formed by subunits called nucleoporins (about 30 different nucleoporins participate in the formation of a single nuclear pore), which in turn, they are grouped into multiprotein sub-complexes located within the nuclear envelope or associated with its external or internal face with respect to the nucleoplasm. The pores are 125 million daltons in molecular weight and are composed of approximately 50 (in yeast) to 100 proteins (in vertebrates). The pores have a total diameter of 100 nm; however, the gap through which the molecules freely diffuse is 9 nm wide due to the presence of regulation systems in the center of the pore. This size allows the free passage of small water-soluble molecules while preventing larger molecules from inappropriately entering or exiting, such as nucleic acids and large proteins. These large molecules must instead be actively transported to the nucleus. The typical nucleus of a mammalian cell has between 3,000 and 4,000 pores along its envelope, each of which contains an octal-symmetric ring structure at the position where the inner and outer membranes meet. they fuse. Anchored to the ring is a structure called the nuclear basket that extends into the nucleoplasm, and a series of filamentous extensions that project into the cytoplasm. Both structures mediate binding to nuclear transport proteins.
Most proteins, ribosome subunits, and some RNAs are transported across pore complexes in a process mediated by a family of transport factors known as caryopherins. Among these are the importins, which are involved in transport in the direction of the nucleus, and those that carry out transport in the opposite direction, known as exportins. Most caryopherins interact directly with their cargo, although some use adapter proteins. Steroid hormones such as cortisol and aldosterone, as well as other small water-soluble molecules involved in cell signaling, can diffuse across the cell membrane and into the cytoplasm, where they bind to proteins that act as nuclear receptors that are conducted to the nucleus. They serve as transcription factors when bound to their ligand. In the absence of ligand many of these receptors function as histone deacetylases that repress gene expression in the organism.
Nuclear foil
In animal cells there are two networks of intermediate filaments that provide mechanical support to the nucleus: the nuclear lamina forms an organized pattern on the inner face of the envelope, while on the outer face this support is less organized. Both networks of intermediate filaments also serve as anchorage sites for chromosomes and nuclear pores.
The nuclear lamina is made up of proteins called lamins or lamin proteins. Like all proteins, they are synthesized in the cytoplasm and later transported into the nucleus, where they are assembled before being incorporated into the pre-existing network. Lamins are also found within the nucleoplasm, where they form another regular structure. known as the nucleoplasmic veil, which is visible during interphase. The structures of the sheets that form the veil bind to chromatin and by disrupting its structure inhibit the transcription of genes that code for proteins.
Like the components of other intermediate filaments, sheet monomers contain an alpha-helical domain, used by two monomers to coil around each other, forming a dimer with a coiled-helical motif. Two of these dimetric structures subsequently join side by side in an antiparallel fashion to form a tetramer called a protofilament. Eight of these protofilaments are arranged laterally to form a filament. These filaments can be assembled or disassembled dynamically, meaning that changes in filament length depend on competing rates of addition and displacement.
Mutations in lamin genes lead to defects in filament assembly known as laminopathies. Of these, the most notable is the family of diseases known as progerias, which give sufferers the appearance of premature aging. The exact mechanism by which the associated biochemical changes give rise to the progeroid phenotype is unknown.
In the event that the nuclear lamina disappears, the nucleus disappears, and in the event that the nucleus reforms, it will be there again; this is a very important property especially in mitosis.
Chromosomes
The cell nucleus contains most of the cell's genetic material in the form of multiple linear DNA molecules known as chromatin, and during cell division this appears in the well-defined form known as a chromosome. A small fraction of the genes are located in other organelles, such as the mitochondria or chloroplasts of plant cells.
It should be noted that the chemical composition of the chromatin fiber lies in DNA segments associated with histone and non-histone proteins, which are duplicated in the S phase of interphase. The histone proteins are: H1, H2A, H2B, H3 and H4; two H2As bind to two H2Bs via the non-histone protein nucleoplasmin, while two H3s and two H4s bind together with the help of the N1 protein, thus forming an octamer, which is surrounded by two and a half turns of DNA to consolidate the nucleosome. The latter, being held by the H1, is now called the chromatosome, and when approaching the M phase these H1 come into direct contact to consolidate a 30 nm diameter solenoid.
There are two types of chromatin: euchromatin is the less compact form of DNA, and contains genes that are frequently expressed by the cell. The other type, known as heterochromatin, is the more compact form, and contains DNA that is transcribes infrequently. This structure is further classified into facultative heterochromatin, which consists of genes that are organized as heterochromatin only in certain cell types or at certain stages of development, and constitutive heterochromatin, consisting of structural components of the chromosome such as telomeres and centromeres. During interphase, chromatin organizes into discrete individual territories, the chromosome territories. Active genes, which are generally found in the euchromatic region of the chromosome, tend to be located at the borders of chromosome territories.
Antibodies to certain types of chromatin organization, particularly nucleosomes, have been associated with various autoimmune diseases such as systemic lupus erythematosus. These are known as antinuclear antibodies (ANA) and have also been observed in concert with multiple sclerosis in in the context of generalized immune dysfunction. As in the aforementioned case of Progeria, the role that antibodies play in inducing the symptoms of autoimmune disease is still unclear.
Nucleolus
The nucleolus is a discrete, densely staining structure found in the nucleus. It is not surrounded by a membrane, so it is sometimes said to be a suborganelle. It is formed around tandem repeats of rDNA, which is the DNA that codes for ribosomal RNA (rRNA). These regions are called nucleolar organizers. The main role of the nucleolus is to synthesize rRNA and assemble ribosomes. The structural cohesion of the nucleolus depends on its activity, since ribosomal assembly in the nucleolus results in a transient association of nucleolar components, facilitating the subsequent assembly of other ribosomes. This model is supported by the observation that rRNA inactivation results in "mixing" of the nucleolar structures.
The first step in ribosomal assembly is the transcription of rDNA by RNA polymerase I, forming a long pre-rRNA precursor. This is cleaved into 5.8S, 18S, and 28S rRNA subunits. Transcription, post-transcriptional processing, and assembly of rRNA take place in the nucleolus, aided by nucleolar small RNA molecules, some of which are derived from introns. spliced messenger RNAs related to ribosomal function. These assembled ribosomal subunits are the largest structures that pass through the nuclear pores.
When viewed under the electron microscope, the nucleolus can be seen to be composed of three distinguishable regions: the fibrillar centers (FCs), surrounded by the dense fibrillar component (DFC), which in turn is bordered by the granular component (GC). rDNA transcription occurs in both the FC and the FC-DFC transition zone, and therefore when rDNA transcription increases, more FC's are observed. Most of the cleavage and modification of rRNAs take place in the DFC, while the last steps involving the assembly of proteins into ribosomal subunits take place in the GC.
Other subnuclear bodies
| Name of structure | Structure diameter |
|---|---|
| Cajal bodies | 0.2-2.0 μm |
| PIKA | 5 μm |
| PML bodies | 0.2-1.0 μm |
| Paraspeckles | 0.2-1.0 μm |
| Speckles | 20-25 nm |
In addition to the nucleolus, the nucleus contains a number of bounded nonmembranous bodies. Among these are the Cajal bodies (coiled bodies), the so-called "Geminis of the coiled bodies" (Gemini of coiled bodies, English), the so-called Polymorphic Interphase Karyosomal Association (PIKA), the Leukemia Corps Promyelocytic leukemia (PMLs), the "paraspeckles" and the "specles de ayuste" or "splice spots" ("splicing speckles" in English). Although little is known about the number of these subnuclear domains, they are significant in that they show that the nucleoplasm is not a uniform mixture, but rather contains organized functional subdomains.
Other subnuclear structures appear as part of pathological processes. For example, the presence of small intranuclear rods has been seen in some cases of nemaline myopathy. This disease is typically caused by mutations in the actin gene, and the rods themselves are made up of the actin produced from such mutant genes, as well as other cytoskeletal proteins.
Cajal Bodies and GEMs
The typical nucleus has 1 to 10 compact structures called Coiled Bodies (CBs), whose diameter measures between 0.2 µm and 2 0.0 µm depending on cell type and species. When viewed under the electron microscope, they resemble tangled tangles of threads, and are dense foci of distribution of the protein coilin. CBs are involved in several different types of functions related to RNA processing, specifically small nucleolar RNA (snoRNA) and small nuclear RNA (snRNA) maturation, and histone mRNA modification.
Similar to the bodies of Cajal are the "Geminis of Coiled Bodies, or GEMs (for its acronym in English of Gemini of Coiled Bodies), whose name is derived from the constellation of Gemini because of its almost twin-like relationship with the Cajal Bodies. GEMs are similar in shape and size to the latter, and in fact are virtually indistinguishable under the microscope. Unlike Cajal bodies, they do not contain snRNPs, but do contain a protein called surviving motor neuron (SMN, for survivor of motor neurons), whose function is related to the biogenesis of snRNP. GEMs are thought to assist CBs in snRNP biogenesis, although it has also been suggested from microscopy evidence that CBs and GEMs are different manifestations of the same structure.
PIKA and PTF domains
PIKA domains, or Polymorphic Interphase Caryosome Associations, were first described in microscopy studies in 1991. Their function was and remains unclear, although they are not thought to be associated with active DNA replication, transcription, or RNA processing. They have been shown to frequently associate with discrete domains defined by dense locations of the transcription factor PTF, which promotes npRNA transcription.
PML Bodies
PML or promyelocytic leukemia protein (PML) bodies are spherical bodies that are scattered in the nucleoplasm, and measuring about 0.2–1.0 µm. They are known by other names, such as "nuclear domain 10" (ND10), "Kremer bodies", and "PML oncogenic domains". They are often seen in the nucleus associated with Cajal bodies. It has been suggested that they play a role in the regulation of transcription. They are usually identified in tumor cells (as in cases of acute promyelocytic leukemia) so they also act as tumor markers.
Paraspeckles
Discovered in 2002, paraspeckles are irregularly shaped compartments of the interchromatin space of the nucleus. First documented in HeLa cells, where they typically occur in 10–30 per nucleus, paraspeckles are now known to they also exist in all human primary cells, transformed cell lines, and tissue sections. Their name is derived from their distribution in the nucleus. The prefix "para" is an apocope of "parallel" and "speckles" (spot or speck, in English) refers to its proximity to the "splicing speckles" or trimming specks.
Paraspeckles are dynamic structures that alter in response to changes in cellular metabolic activity. They are transcription dependent, and in the absence of RNA Pol II transcription, the paraspeckles disappear, and all of the associated proteins that compose it (PSP1, p54nrb, PSP2, CFI(m)68, and PSF) form a perinucleolar plug in the paraspeckles. crescent shape in the nucleolus. This phenomenon is manifested during the cell cycle, in which they are present in interphase and throughout mitosis, except in telophase. During telophase, when the two daughter nuclei form, there is no transcription by RNA polymerase II, so the protein components form a perinucleolar plug instead.
Speckles
Sometimes called clusters of interchromatin granules or compartments of splicing factors, speckles are rich in snRNA from splicing and other splicing proteins processes that are needed in the processing of pre-mRNA. Due to the variable requirements of the cell, the composition and location of these bodies change according to the transcription of mRNA and regulation via phosphorylation of specific proteins.
Cleavage Bodies
Called Cleavage bodies, in English, they are usually found associated with Cajal bodies, with a diameter of 0.2 to 1.0 μm and a number of 1-10 per nucleus. Unlike other nuclear bodies, they appear only during certain periods of the cell cycle. Some of these contain the cleavage and polyadenylation specificity factor (CPSF-100) complex, and can be seen predominantly during S and G phases, while those containing CstF-64-containing polyadenylation factor are mainly observed in S phase. They are associated with the histone gene cluster.
DDX1 Bodies
DDX1 bodies are aggregates of the DDX1 protein, belonging to the family of RNA helicases that contain the "DEAD box" motif, they are found in a number that varies from two to four. Since these bodies appear to be recruited to sites of DNA damage that is hybridizing to DNA, it appears that these bodies play a role in repairing areas of double-strand breaks, facilitating pattern-guided repair. of transcriptionally active regions of the genome.
Function
The main function of the cell nucleus is to control gene expression and mediate DNA replication during the cell cycle. The nucleus provides a site for transcription in the cytoplasm, allowing levels of regulation not available in prokaryotes. It has different functions:
- In the nucleus the genes are stored in chromosomes (during mitosis) or chromatin (during the interphase)
- Organizes genes in chromosomes which allows cell division
- Transports regulatory factors through nuclear pores
- It produces courier ribonucleic acid (ARNm) that encodes proteins.
- It produces pre-ribosomas (ARNr) in the nucleus.
Cellular compartmentalization
The nuclear envelope allows the nucleus to control its contents and separate it from the rest of the cytoplasm when necessary. This is important for controlling processes on either side of the nuclear membrane. In some cases, when a cytoplasmic process needs to be restricted, a key player is removed to the nucleus, where it interacts with transcription factors to repress the production of certain enzymes in the pathway. This regulatory mechanism takes place in the case of glycolysis, a cellular pathway in which glucose is used to produce energy. Hexokinase is the enzyme responsible for the first step of glycolysis, producing glucose-6-phosphate from glucose. At high concentrations of fructose-6-phosphate, a molecule that is subsequently formed from glucose-6-phosphate, hexokinase is removed to the nucleus by a regulatory protein, where it forms a complex with other nuclear proteins that represses the transcription of fructose-6-phosphate. genes involved in glycolysis.
To control which genes must be transcribed, the cell prevents the physical access of some transcription factors responsible for regulating gene expression until they are activated by other signaling pathways. This prevents even small levels of inappropriate gene expression from occurring. For example, in the case of genes controlled by NF-κB, which are involved in most inflammatory responses, transcription is induced in response to a cellular signaling cascade such as that initiated by the signaling molecule TNF-β. α binding to a cell membrane receptor, resulting in the recruitment of signaling proteins and ultimately activation of the transcription factor NF-κB. A nuclear localization signal possessed by the NF-κB protein allows it to be transported through the nuclear pore to the nucleus, where it stimulates the transcription of target genes.
Compartmentalization allows the cell to prevent translation of unspliced mRNA. The mRNA contains introns that must be removed before being translated to produce functional proteins. Splicing takes place inside the nucleus before the mRNA can access ribosomes for translation. Without the nucleus, ribosomes would translate raw, newly transcribed mRNA, producing misfolded and misshapen proteins.
Gene Expression
Gene expression primarily involves transcription, in which DNA is used as a template to produce RNA. In the case of protein-coding genes, the RNA generated by this process is messenger RNA (mRNA), which then needs to be translated by ribosomes to form a protein. Since ribosomes are located outside the nucleus, the synthesized mRNA must be exported.
Since the nucleus is the place where transcription occurs, it is endowed with a set of proteins that are either directly involved in this process or in its regulation. These include helicases, which unwind the double-stranded DNA molecule to facilitate access by the synthesis machinery, RNA polymerase, which synthesizes RNA from the DNA template, topoisomerase, which varies the amount of supercoiling of the DNA, as well as a wide variety of transcription factors that regulate gene expression.
Pre-mRNA processing
Newly synthesized mRNA molecules are known as primary transcripts or pre-mRNA. They must then undergo post-transcriptional modification in the nucleus before being exported to the cytoplasm. The mRNA that appears in the nucleus without these modifications ends up degraded instead of being used for translation in the ribosomes. The three main modifications are: The one at the extreme 5' (5' caping), polyadenylation of the 3' end and RNA splicing. While in the nucleus, the pre-mRNA associates with various proteins in complexes known as nuclear heterogeneous ribonucleoproteins, or hnRNPs. The addition of the 5' it takes place at the time of transcription and is the first step in post-transcriptional modifications. The 3' it is only added once the transcript is complete.
RNA splicing, carried out by a complex called a spliceosome, is the process by which introns are removed from pre-mRNA, leaving only the exons connected to form a single continuous molecule. This process normally ends after the previous two, but can begin before synthesis is complete in transcripts with many exons. Many pre-mRNAs, including those encoding antibodies, can be spliced in multiple ways to produce different mature mRNAs, thereby encoding different protein sequences. This process is known as alternative splicing, and it allows the production of a wide variety of proteins from a limited amount of DNA.
Dynamics and regulation
Nuclear transport
The transport of molecules to the exterior and interior of the nucleus can be carried out thanks to the fact that in all eukaryotic cells the nuclear envelope is perforated by nuclear pores, made up of large multiprotein complexes. The entry and exit of large molecules from the nucleus is tightly controlled by the nuclear pore complexes. Although small molecules can enter the nucleus unregulated, macromolecules such as RNA and proteins require caryopherins called importins to enter the nucleus, and exportins to leave. The loaded proteins that must be translocated from the cytoplasm to the nucleus contain short amino acid sequences known as nuclear localization signals that are bound to importins, while those transported from the nucleus to the cytoplasm have exportin-bound nuclear export signals. The ability of importins and exportins to transport their cargo is regulated by GTPases, enzymes that hydrolyze GTP, releasing energy. The key GTPase in nuclear transport is Ran, which can bind either GTP or GDP (guanosine diphosphate), depending on whether it is located in the nucleus or the cytoplasm. While importins rely on Ran-GTP to dissociate from their cargo, exportins require Ran-GTP to bind their cargo.
Location signs necessary for transport
Nuclear localization signals make the flow of proteins from the cytosol to the nucleus selective. These signals are only present in nuclear proteins, they consist of a short sequence between 4 and 8 amino acids. When there is nuclear import this signal is called nuclear localization signal (NLS), and when there is nuclear export it is called nuclear export signal (NES).
There are two types of NLS: one-party and two-party. Monopartite NLS are made up of a single group of basic residues and bipartite NLS are made up of two groups of lysine and arginine residues. These types of signals are specifically recognized by Importin α and the proteins that contain them are transported to the nucleus by the Importin α/Importin β1 heterodimer. On the other hand, NES are short sequences of hydrophobic amino acids, mainly leucines.
These nuclear localization signals, which are located in the nuclear pores, are joined by one or more nucleoporins, which are cytosolic proteins that contain N-acetylglucosamine, a simple sugar that helps their identification through the use of lectins and specific antibodies. Nucleoporins help by directing the nuclear protein toward the center of the pore complex, where it binds to fibrils that extend toward the cytosol and project from the ring of the complex. These fibrils guide nuclear proteins to the center of the pore complex, where they are actively transported into the nuclear interior by a process that requires GTP hydrolysis.
Karyopherins
They are proteins that mediate transport through the nuclear pore complex. Their classification depends on the direction of transport for which they were initially described; they have been classified as importin and exportin.
Most of the importins belong to the β importin superfamily and are responsible for regulating the transport of most proteins and different RNA species, except mRNA.
Nuclear export
It occurs in conditions of high concentration of Ran-GTP, it recognizes a protein that contains a NES (nuclear export signal) together with a Ran-GTP molecule. The complex is then able to interact with the nuclear pore complex and pass through it into the cytoplasm. Once there, other Ran proteins promote Ran's GTPase activity, which hydrolyzes GTP into Ran-GDP. Hydrolysis produces a conformational change in Ran, producing the disassembly of the exportin-cargo, leaving the cargo free in the cytoplasm. The Ran-GDP and exportin molecules are recycled for a new transport cycle.
Specialized export proteins serve the translocation of mature mRNA and tRNA into the cytoplasm after post-transcriptional modification is complete. This quality control mechanism is important because of the central role of these molecules in protein translation. Inappropriate expression of a protein due to incomplete exon exonization or improper amino acid incorporation could have negative consequences for the cell. Therefore, completely unchanged RNA that reaches the cytoplasm is degraded instead of being used in translation.
Nuclear import
Nuclear import depends on importin binding its cargo in the cytoplasm and transporting it through the nuclear pore to the nucleus. Importins interact in the cytoplasm, under conditions of low RanGTP concentration, with the protein with an NLS (nuclear localization signal), and it enters the interior of the nucleus by association with proteins of the nuclear pore complex. Once in the nucleoplasm, the presence of high levels of RanGTP causes the destruction of the importin-cargo complex, releasing the cargo inside the nucleus. Importin α is transported back to the cytoplasm through their interaction, restarting the process again.
Regulation of transport between the nucleus and the cytosol
Transport between the cytosol and the nucleus can be regulated by inactivating the nuclear localization signal of nuclear proteins by phosphorylation or when these proteins bind to inhibitory cytosolic proteins that retain them in the cytosol through interactions with the cytoskeleton or with specific organelles, or mask their nuclear localization signals. However, when the cell receives the necessary stimulus, the nuclear protein is released and is transported to the nucleus.
In a similar way, the export of RNA from the nucleus can be controlled. Like active import into the kernel, export requires a signal. Nuclear export signals are likely to be located in the protein subunits of such complexes, and to be activated after proper assembly with the RNA components.
For all this, it can be concluded that the mechanism of transport of macromolecules through the nuclear pore is very different from the mechanism that occurs through the membranes of other organelles, since nuclear transport does not occur by a protein transporter that crosses a or more lipid bilayers but through a pore with a regulated aqueous channel. Also, while nuclear proteins are transported through the pores maintaining their fully folded conformation, in transport to other organelles, the proteins have to unfold. Finally, nuclear localization signals are not removed after transport to the nucleus, since nuclear proteins have to be imported into the nucleus several times after each cell division. But, when a protein has been imported into any other membranous organelle, the signal peptide is often removed after protein translocation.
Assembly and disassembly
During its lifetime, a nucleus can disassemble, either in the course of cell division, or as a consequence of apoptosis, a regulated form of cell death. During these events, the structural components of the core—the shell and the lamina—are systematically degraded.
During the cell cycle, the cell divides to form two cells. For this process to be possible, each of the new daughter cells must acquire a complete set of genes, a process that requires replication of the chromosomes as well as segregation into separate sets. This occurs when already replicated chromosomes, the daughter chromatids, attach to microtubules, which in turn attach to different centrosomes. Daughter chromatids can be cleaved to separate locations in the cell. However, in many cells the centrosome is located in the cytoplasm, outside the nucleus, so the microtubules would be unable to attach to the chromatids in the presence of the nuclear envelope. Thus, in the early stages of the cell cycle, beginning in prophase and up to near prometaphase, the nuclear membrane dismantles. Similarly, during the same period, the nuclear lamina disassembles, a process that is regulated by phosphorylation of the lamins. Towards the end of the cell cycle it reforms. the nuclear membrane, and around the same time, the nuclear lamina reassembles by dephosphorylating the lamellar proteins.
Apoptosis is a controlled process in which the structural components of the cell are destroyed, resulting in cell death. Changes associated with apoptosis directly affect the nucleus and its contents, for example in the condensation of chromatin and the disintegration of the nuclear envelope and lamina. The destruction of the lamina networks is controlled by specialized apoptotic proteases called caspases, which disintegrate the nuclear lamina and thereby degrade the structural integrity of the nucleus. Disintegration of the nuclear lamina is sometimes used in laboratories as an indicator of caspase activity in assays of early apoptotic activity. Cells expressing caspase-resistant lamins are deficient in apoptosis-related nuclear changes, thus which suggests that lamins play an important role in initiating events leading to apoptotic degradation of the nucleus. Inhibition of nuclear lamina assembly itself is itself an inducer of apoptosis.
The nuclear envelope acts as a barrier that prevents DNA or RNA viruses from entering the nucleus. Some viruses need access to proteins within the nucleus in order to replicate or assemble. DNA viruses, such as herpesviruses, replicate and assemble in the cell nucleus, and bud through the inner nuclear membrane. This process is accompanied by the disassembly of the nuclear lamina on the nuclear side of the inner membrane.
Anucleated and polynucleated cells
Although most cells have a single nucleus, some cell types do not have one, while others have multiple nuclei. This may be a normal process, such as red blood cell maturation, or the result of faulty cell division.
Enucleated cells lack a nucleus and are therefore incapable of dividing to produce daughter cells. The best known case of an anucleated cell is the mammalian erythrocyte, which also lacks other organelles such as mitochondria, and they serve primarily as vehicles for transporting oxygen from the lungs to the tissues. Red blood cells mature through erythropoiesis in the bone marrow, where they lose their nucleus, organelles, and ribosomes. The nucleus is expelled during the process of differentiation from erythroblast to reticulocyte, which is the immediate precursor of the mature erythrocyte. Mutagens can induce the release of some "micronucleated" immature erythrocytes; into the bloodstream. Anucleated cells can also arise from defective cell division in which one daughter cell lacks a nucleus, while the other has two.
Polynucleated cells contain multiple nuclei. Most protozoa of the class Acantharea, and some fungi that form mycorrhizae, have naturally polynucleated cells. Other examples would be intestinal parasites of the genus Giardia, which have two nuclei in each cell. In humans, skeletal muscle contains cells, called myocytes, which become polynucleated during development. The resulting arrangement of nuclei in the peripheral region of the cell allows for maximum intracellular space for the myofibrils. Multinucleated cells can also be abnormal in humans. For example, those that arise from the fusion of monocytes and macrophages, known as multinucleated giant cells, can sometimes be seen accompanying inflammation, and are also involved in tumor formation.
Evolution
As the best defining feature of the eukaryotic cell, the evolutionary origin of the nucleus has been the subject of much speculation. Among the proposed theories, four can be considered as the main ones, although none of them has found wide support.
Endosymbiotic theories
The theory known as the "syntrophic model" proposes that a symbiotic relationship between archaea and bacteria created the first nucleated eukaryotic cell. The hypothesis is established that the symbiosis took place when an ancient archaea similar to the current methanogens were invaded and parasitized by bacteria similar to the current myxobacteria, finally forming the primitive nucleus. This theory is analogous to the accepted theory of the origin of eukaryotic mitochondria and chloroplasts, which are thought to have developed by a similar endosymbiont relationship between protoeukaryotes and aerobic bacteria. The archean origin of the nucleus is supported by the fact that both Archaea and eukaryotes have similar genes on certain proteins, including histones. Noting that myxobacteria are motile, can form multicellular complexes, and possess eukaryotic-like G proteins, a bacterial origin of the eukaryotic cell can also be accepted. A similar proposal states that a eukaryotic-like cell, the chronocyte, it appeared first, and subsequently phagocytosed archaea and bacteria to give rise to the nucleus and eukaryotic cell.
A more controversial model, known as viral eukaryogenesis claims that many features of the eukaryotic cell such as the presence of a membrane-continuous nucleus arose from infection of a prokaryotic ancestor by a large DNA virus (possibly from a large DNA Nucleocytoplasmic Virus). This is suggested on the basis of similarities between eukaryotes and viruses such as linear DNA strands, "caping" from the 5' of the mRNA and the strong binding to DNA proteins (making the histones analogous to the viral envelope). One version of this proposal suggests that the nucleus evolved in concert with phagocytosis to give rise to a primitive cellular predator. Another variant proposes that eukaryotes arose from poxvirus-infected primitive archaea, based on similarity of modern DNA polymerases between these and eukaryotes. It has been suggested that the unresolved question of the evolution of sexuality may be related to the viral eukaryogenesis hypothesis.
Non-endosymbiotic theories
This model proposes that proto-eukaryotic cells evolved from bacteria without a symbiont stage. This model is based on the existence of a modern bacterium belonging to the planctomycete phylum that has a nuclear structure with primitive pores and other structures compartmentalized by membrane.
Finally, a very recent proposal suggests that the traditional variants of endosymbiont theories are insufficient to explain the origin of the eukaryotic nucleus. This model, termed the exomembrane hypothesis, suggests that the nucleus originated instead from an original ancestral cell that developed a second outer cell membrane. The inner membrane enclosing the original cell then became the nuclear membrane evolving to develop increasingly elaborate pore structures for the passage of internally synthesized cellular components, such as ribosomal subunits.
Contenido relacionado
Category:Molecular biologists
Ovary
Ethology