Catalysis
catalysis is the process by which the speed of a chemical reaction is increased, due to the participation of a substance called catalyst; those that deactivate catalysis They are called inhibitors. An important characteristic is that the mass of the catalyst does not change during the chemical reaction, which differentiates it from a reactant, whose mass decreases throughout the reaction.
In the synthesis of many of the most important industrial chemicals there is a catalysis, since this can reduce the time required. Catalyst poisoning, which is generally an unwanted process, is also used in the chemical industry. For example, in the reduction of ethyne to ethene, the palladium (Pd) catalyst is partially "poisoned" with lead(II) acetate, Pb(CH3COO)2. Without catalyst deactivation, the ethene produced would subsequently be reduced to ethane.
General information
Catalysis is involved in many industrial processes. Likewise, most "biologically" significant processes are catalyzed. Catalysis research is one of the main fields in applied science and involves many areas of chemistry, especially organometallic chemistry and materials science. Catalysis is important to many aspects of environmental science, for example, the automotive catalytic converter and the dynamics of the ozone hole. Catalytic reactions are preferred in environmentally friendly green chemistry due to the reduced amount of waste generated rather than stoichiometric reactions in which all reactants are consumed and more by-products are formed. The most common catalyst is the proton (H+). Many transition metals and transition metal complexes are used in catalysis. Catalysts called enzymes are important in biology.
The catalyst works by providing an alternative reaction path to the reaction product. The rate of the reaction increases as this alternative pathway has a lower activation energy than the non-catalyst mediated reaction pathway. The disproportionation of hydrogen peroxide to give water and oxygen is a reaction that is strongly affected by catalysts:
- 2 H2O2 → 2 H2O + O2
This reaction is favored, in the sense that the reaction products are more stable than the starting material, however, the uncatalyzed reaction is slow. The decomposition of hydrogen peroxide is in fact so slow that hydrogen peroxide solutions are commercially available. After the addition of a small amount of manganese dioxide, hydrogen peroxide reacts rapidly according to the above equation. This effect is easily seen by the effervescence of oxygen. Manganese dioxide can be recovered unchanged, and reused indefinitely, and is therefore not consumed in the reaction. Consequently, manganese dioxide catalyzes this reaction.
Features
The general characteristic of catalysis is that the catalytic reaction has a smaller free energy change from the limiting stage to the transition state than the corresponding uncatalyzed reaction, resulting in a higher reaction rate at the same temperature. However, the mechanical origin of catalysis is complex.
Catalysts can favorably affect the reaction environment, for example, acid catalysts for reactions of carbonyl compounds form specific intermediates that do not occur naturally, such as osmium esters in the dihydroxylation of alkenes catalyzed by osmium tetroxide, or breaking down reactants to reactive forms, such as atomic hydrogen in catalytic hydrogenation.
Kinetically, catalytic reactions behave like typical chemical reactions, that is, the reaction rate depends on the frequency of contact of the reactants in the rate-determining step (see Arrhenius equation). Normally, the catalyst participates in this slow stage, and the rates are limited by the amount of catalyst. In heterogeneous catalysis, diffusion of reactants to the interface and diffusion of products from the interface may be the rate-determining step. Similar events related to substrate binding and product dissociation apply in homogeneous catalysis.
Although catalysts are not consumed by the reaction itself, they can be inhibited, deactivated, or destroyed by secondary processes. In heterogeneous catalysis, typical side processes include coking, where the catalyst is covered by polymeric side products. Furthermore, heterogeneous catalysts can dissolve in solution in a solid-liquid system or evaporate in a solid-gas system.
General principles of catalysis
Typical Mechanism
Catalysts generally react with one or more of the reactants to form intermediate products that subsequently lead to the final reaction product. In the process the catalyst is regenerated. The following scheme is typical of a catalytic reaction, where C represents the catalyst, X and Y are the reactants, and Z is the product of the reaction of X with Y:
- X C → XC (1)
- Y + XC → XYC (2)
- XYC → CZ (3)
- CZ → C + Z (4)
Although the catalyst is consumed by reaction 1, it is subsequently produced by reaction 4, so the overall reaction is:
- X + Y → Z
Since catalyst is regenerated in a reaction, small amounts of catalyst are often enough to increase the rate of a reaction. However, in practice catalysts are sometimes consumed in secondary processes.
As an example of this process, in 2008, Danish researchers first revealed the sequence of events when oxygen and hydrogen combine on the surface of titanium dioxide (TiO2, or titania) to produce water. Using a series of time-lapse scanning tunneling microscopy images, they determined that the molecules undergo adsorption, dissociation, and diffusion before reacting. The intermediate reaction states were: HO2, H2O2, then H3O 2 and the final product of the reaction (dimers of the water molecule), after which the water molecule is desorbed from the catalyst surface.
Catalysis and energetics of the reaction
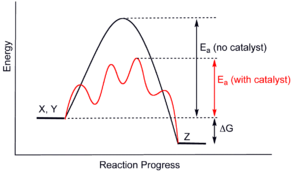
Catalysts work by providing an (alternative) mechanism involving a different transition state and lower activation energy. Therefore, more molecular collisions have the necessary energy to reach the transition state. Consequently, catalysts allow reactions that would otherwise be blocked or slowed down by a kinetic barrier. The catalyst can increase reaction rate or selectivity, or allow the reaction to occur at lower temperatures. This effect can be illustrated with a Boltzmann distribution and an energy profile diagram.
Catalysts do not change the yield of a reaction: they do not have an effect on the chemical equilibrium of a reaction, because the rate of both the forward reaction and the conversely, they are affected (see also thermodynamics). The fact that a catalyst does not change the equilibrium is a consequence of the second law of thermodynamics. Suppose there is a catalyst that changes the equilibrium. The introduction of the catalyst into the system would result in the reaction to go back to equilibrium, producing energy. The production of energy is a necessary result, since reactions are spontaneous if and only if Gibbs free energy is produced, and if there is no energy barrier there is no need for a catalyst. Consequently, removal of the catalyst would also result in a reaction, producing energy; that is, both the addition and its inverse process, the elimination, would produce energy. Thus, a catalyst that could change the equilibrium would be perpetually mobile, in contradiction to the laws of thermodynamics.
If a catalyst changes the equilibrium, then it must be consumed as the reaction progresses, and is therefore also a reactant. Some illustrative examples are the base-catalyzed hydrolysis of esters, where the produced carboxylic acid immediately reacts with the basic catalyst, thus shifting the equilibrium of the reaction towards hydrolysis.
The SI derived unit for measuring the catalytic activity of a catalyst is the katal, which is equal to moles per second. The activity of a catalyst can be described by the number of conversions, or TON (from English turn over number), and the catalytic efficiency by the frequency of conversions, TOF (from English turn over frequency). The biochemical equivalent is the unit of enzyme activity. For more information on the efficiency of enzyme catalysis, see the article on enzyme catalysis.
The catalyst stabilizes the transition state more than stabilizes the starting material. It decreases the kinetic barrier by decreasing the difference of energy between the initial material and the transition state.
Typical Catalytic Materials
The chemical nature of catalysts is as diverse as the catalysis itself, although some generalizations can be made. Protic acids are probably the most widely used catalysts, especially for many reactions involving water, including hydrolysis and its reverse. Multifunctional solids are often catalytically active, for example zeolites, alumina, and certain forms of graphitic carbon. Transition metals are often used to catalyze redox reactions (oxygenation, hydrogenation). Many catalytic processes, especially those involving hydrogen, require platinum group metals.
Some of the so-called catalysts are actually pre-catalysts. Precatalysts become the catalyst in the course of the reaction. For example, the Wilkinson catalyst RhCl(PPh3)3 loses a triphenylphosphine ligand before entering the true catalytic cycle. Precatalysts are easier to store, but are easily activated in situ. Due to this preactivation step, many catalytic reactions involve an induction period.
Chemical species that improve catalytic activity are called co-catalysts or promoters, in cooperative catalysis.
Types of catalysis
Catalysts can be homogeneous or heterogeneous, depending on whether a catalyst exists in the same phase as the substrate. Biocatalysts are often viewed as a separate group.
Enzyme catalysts
They are those that carry out processes through enzymes, these are more used in industrial areas, one of them is pharmaceutical, in which an example is for the production of insulin, which is obtained by using the enzyme of the bacteria E. coli.
Heterogeneous Catalysts
Heterogeneous catalysts are those that act in a different phase than the reactants. Most heterogeneous catalysts are solids that act on substrates in a liquid or gaseous reaction mixture. Various mechanisms are known for reactions on surfaces, depending on how adsorption is carried out (Langmuir-Hinshelwood, Eley-Rideal, and Mars-van Krevelen). The total surface area of the solid has a major effect on the rate of adsorption. reaction. The smaller the catalyst particle size, the greater the surface area for a given mass of particles.
For example, in the Haber process, finely divided iron serves as a catalyst for the synthesis of ammonia from nitrogen and hydrogen. The reactant gases are adsorbed on the "active sites" of the iron particles. Once adsorbed, the bonds within the reacting molecules suffer, and new bonds are formed between the generated fragments, partly due to their proximity. In this way the particularly strong triple bond in nitrogen is weakened and the hydrogen and nitrogen atoms combine faster than would be the case in the gas phase, thereby increasing the rate of reaction.
Heterogeneous catalysts are typically "supported," which means that the catalyst is dispersed in a second material that improves efficiency or minimizes its cost. Sometimes the catalyst support is more than a surface on which the catalyst is broadcast to increase the surface area. More often, the support and the catalyst interact, affecting the catalytic reaction.
Homogeneous catalysts
Normally homogeneous catalysts are dissolved in a solvent with the substrates. An example of homogeneous catalysis involves the influence of H+ on the esterification of esters, for example, methyl acetate from acetic acid and methanol. For inorganic chemists, homogeneous catalysis is often synonymous with organometallic catalysts.
Electrocatalysts
In the context of electrochemistry, specifically in fuel cell engineering, catalysts containing various metals are used to improve the rates of the half-reactions that make up the fuel cell. A common type of fuel cell electrocatalyst is based on platinum nanoparticles that are supported on slightly larger particles of carbon. When this platinum electrocatalyst is in contact with one of the electrodes in a fuel cell, the rate of reduction of oxygen to water (or hydroxide or hydrogen peroxide) increases.
Organocatalysis
While transition metals sometimes attract the most attention in the study of catalysis, organic molecules that do not contain metals can also possess catalytic properties. Organic catalysts typically require a higher loading (or amount of catalyst per unit amount of reactant) than transition metal based catalysts, but these catalysts are often commercially available in large quantities, helping to reduce costs. In the early 2000s, organocatalysts were considered a "new generation" and were competitors to traditional metal-containing catalysts. Enzymatic reactions operate through the principles of organic catalysis.
Nanocatalysis
The principle of nanocatalysis is based on the premise that catalytic materials applied at the nanoscale have better properties, compared to what they exhibit at the macroscale.
Importance of catalysis
It is estimated that 90% of all commercially produced chemicals involve catalysts at some stage in their manufacturing process. In 2005, catalytic processes generated nearly $900 billion worth of products worldwide. The catalysis is so pervasive that the subareas are not easily classifiable. Some areas of particular concentration
Energy Processing
Petroleum refining makes heavy use of catalysis for alkylation, catalytic cracking (breaking long-chain hydrocarbons into smaller pieces), naphtha reforming, and steam reforming (conversion of hydrocarbons to synthesis gas). Even exhaust gases from burning fossil fuels are treated through catalysis: catalytic converters, typically composed of platinum and rhodium, break down some of the most harmful byproducts of automobile exhaust.
- 2 CO + 2 NO → 2 CO2 + N2
With respect to synthetic fuels, an old but important process is the Fischer-Tropsch synthesis of hydrocarbons from syngas, which in turn is processed through the water-gas shift reaction, catalyzed by iron. Biodiesel and related biofuels require processing through both inorganic catalysts and biocatalysts.
Fuel cells are based on catalysts for both anodic and cathodic reactions.
Bulk Chemicals
Some of the chemicals produced on a large scale are produced through catalytic oxidation, often using oxygen. Some examples are nitric acid (from ammonia), sulfuric acid (from sulfur dioxide to sulfur trioxide by the lead chamber process), terephthalic acid from p-xylene, acrylic acid from propylene or propane, and acrylonitrile from propane and ammonia.
Many other chemicals are generated by large-scale reduction, often through hydrogenation. The largest scale example is ammonia, which is prepared through the Haber process from nitrogen. Methanol is prepared from carbon monoxide.
Bulk polymers derived from ethylene and propylene are often prepared via Ziegler-Natta catalysis. Polyesters, polyamides, and isocyanates are obtained through acid-base catalysis.
Most carbonylation processes require metal catalysts, examples include the synthesis of acetic acid via the Monsanto process and hydroformylation.
Fine chemistry
Many fine chemicals are prepared via catalysis, methods include those of heavy industry as well as more specialized processes that would be prohibitively expensive on a large scale. Some examples are olefin metathesis using the Grubbs catalyst, the Heck reaction, and the Friedel-Crafts reaction.
Because most bioactive compounds are chiral, many pharmaceuticals are produced by enantioselective catalysis.
Food processing
One of the most obvious applications of catalysis is the hydrogenation (reaction with hydrogen gas) of fats using nickel as a catalyst to produce margarine. Many other food products are prepared via biocatalysis (see below)..
Biology
In nature, enzymes are catalysts in metabolism and catabolism. Most biocatalysts are protein-based, i.e. enzymes, but other classes of biomolecules also exhibit catalytic properties including ribozymes, and synthetic deoxyribozymes.
Biocatalysts can be considered as intermediate between homogeneous and heterogeneous catalysts, although strictly speaking soluble enzymes are homogeneous catalysts and membrane-bound enzymes are heterogeneous. Several factors affect the activity of enzymes (and other catalysts), including temperature, pH, enzyme concentration, substrate, and products. A particularly important reactant in enzymatic reactions is water, which is the product of many bond-forming reactions and a reactant in many bond-breaking processes.
Enzymes are used to make basic chemicals, including corn syrup and acrylamide.
In the environment
Catalysis has an impact on the environment by increasing the efficiency of industrial processes, but catalysis also plays a direct role in the environment. A notable example is the catalytic role of free radicals in ozone destruction. These radicals are formed by the action of ultraviolet radiation on chlorofluorocarbons (CFCs).
- Cl· + O3 → ClO· + O2
- ClO· + O· → Cl· + O2
Etymology and history
In a general sense, anything that increases the speed of a process is a "catalyst," a term derived from the Greek, meaning "break away", "unleash", or "pick up". The phrase catalyzed processes was coined by Jöns Jakob Berzelius in 1836 to describe reactions that are accelerated by substances that remain unchanged after the reaction. Other early chemists involved in catalysis were Alexander Mitscherlich who referred to contact processes and Johann Wolfgang Döbereiner who spoke of contact action and whose hydrogen-based igniter and a platinum sponge became a great commercial success in the 1820s. Humphry Davy discovered the use of platinum in catalysis. In the 1880s, Wilhelm Ostwald at the University of Leipzig began a systematic investigation of reactions that were catalyzed by the presence of acids and bases, and found that chemical reactions occur at finite rates and that these rates can be used To determine the strength of acids and bases. For this work, Ostwald was awarded the 1909 Nobel Prize in Chemistry.
Inhibitors, poisons and promoters
Substances that reduce the action of catalysts are called catalyst inhibitors if they are reversible, and catalyst poisons if they are irreversible. Promoters are substances that increase catalytic activity, particularly when they are not catalysts themselves.
The inhibitor can modify selectivity as well as speed. For example, in the reduction of ethyne to ethene, the catalyst is palladium (Pd), partially "poisoned" with lead(II) acetate (Pb(CH3COO)2). Without catalyst deactivation, the ethylene produced will be further reduced to ethane.
The inhibitor can produce this effect by, for example, selectively poisoning only certain types of active sites. Another mechanism is the modification of the geometry of the surface. For example, in hydrogenation operations, large metal-surfaced plates function as hydrogenolytic catalysis sites while the sites that catalyze the hydrogenation of unsaturates are smaller. Thus, a poison that covers the surface randomly tends to reduce the number of large uncontaminated plates, but leaves proportionally more small sites free, thus switching hydrogenation versus selective hydrogenolysis. Many other mechanisms are also possible.
Energy diagrams
The figure shows a diagram of a catalyzed reaction, showing how the energy (E) of the molecules participating in the reaction varies during the reaction process (time, t). All molecules contain a certain amount of energy, which depends on the number and type of bonds present in it. The substrates or reagents (A and B) have a certain energy, and the product(s) (AB in the graph), another.
If the total energy of the substrates is greater than that of the products (for example as shown in the diagram), an exothermic reaction, and excess energy is released as heat. Conversely, if the total energy of the substrates is less than that of the products, energy needs to be taken from outside for the reaction to take place, which is called an endothermic reaction.
As substrate molecules move closer to react, they lose stability (using an anthropomorphic analogy, molecules 'like' to keep their living space, and meddling is not welcome). The instability manifests itself as an increase in the energy of the system (it is the energy peak that you see in the diagram). When the substrates become products, the molecules separate and relax again, and the whole stabilizes.
Enzymes catalyze reactions by stabilizing the reaction intermediate, so that the "peak" energy needed to go from substrates to products is less. The end result is that there are many more substrate molecules colliding and reacting to produce the products, and the reaction generally proceeds faster. A catalyst can catalyze both endothermic and exothermic reactions, because in both cases it is necessary to overcome an energy barrier. The catalyst (E) creates a microenvironment in which A and B can reach the intermediate state (A...E...B) more easily, reducing the amount of energy needed (E2). As a result, the reaction is easier, optimizing the rate of said reaction.
Catalysts do not alter the chemical equilibrium of the reaction in any way.
Contenido relacionado
Pyrimidine
Aluminosilicate
Acetobacteria