Casein

Casein (from the Latin caseus, "cheese") is a phosphoprotein (a type of heteroprotein) present in milk and some its derivatives (fermented products such as yogurt or cheese). In milk, it is found associated with calcium (calcium phosphate), forming aggregates called casein micelles. Table 1 shows the content of this protein in the milk of different mammal species.
| component | species | |||
|---|---|---|---|---|
| human | Bovina | ovina | caprine | |
| proteins (% of total dairy) | 1.3-1.5 | 3.2-3.5 | 5.4-6.0 | 3.1-4.0 |
| caseins (% of total protein) | 44.9 | 82.5 | 84.8 | 81.3 |
From the data in this table it can be deduced that the milk of the human species not only contains a lower proportion of proteins, but also contains less caseins than the other species.
Characteristics of caseins


Caseins are a heterogeneous group of proteins, so it is difficult to establish a definition. However, all the proteins included in what is called casein have a common characteristic: they precipitate when milk is acidified to pH 4.6. For this reason, casein is also often called insoluble milk protein. On the other hand, and although the proteins called caseins are specific to each species, they are classified into the following large groups according to their electrophoretic mobility: αs1-casein, αs2-casein, β-casein and κ-casein (see Figure 1). The latter is of special interest in the cheese industry, since its enzymatic hydrolysis by rennet (the enzyme chymosin) reduces the hydrophilicity of the casein micelles and causes their aggregation. The k-casein fragment that is maintained in the micelle is known as para-κ-casein.
Chemistry and physics of caseins
Unlike many other proteins, caseins do not precipitate easily due to heat, since, being rich in proline, they have few sections with a defined primary structure. At acidic pH, the micelles lose their charge and their ability to repel each other, and the caseins aggregate. When obtained industrially, this material is called acid casein. Coagulation mediated by acidification is the process that occurs in yogurts. Enzymatic coagulation occurs in the curd. In cheeses, depending on the type, a combination of both effects can occur.
The characteristics of cow's milk caseins are summarized in Table 2. The amino acid sequence (see Figure 2) of casein contains an unusual number of proline amino acid residues: among 10 in αs2 -casein and 35 in β-casein. Caseins are relatively hydrophobic (poorly soluble in water).
Another interesting fact, used to separate caseins from the rest of the milk proteins through their precipitation, is that their average isoelectric point (pI) is 4.6. At this pH, caseins are at their point of lowest solubility due to the reduction of intermolecular repulsions, which is why they precipitate (colloquially, they are said to coagulate). Now, the pH is different for each of the casein fractions, since it varies between 4.44-4.97, for αs1-casein, and 5.3-5.8, in the genetic variant B of κ-casein.
| Feature | home | |||
|---|---|---|---|---|
| αs1 | αs2 | β | κ | |
| Milk concentration (g/L) | 12-15 | 3-4 | 9-11 | 2-4 |
| Genetic variants | B and C | A | A1 and A2 | A and B |
| Molecular mass | 23.545 - 23.615 | 25.226 | 23.983 - 24.023 | 19.006 - 19.037 |
| Isoelectric point (pI) | 4,44 - 4,76 | ... | 4.83 - 5.07 | 5.45 - 5.77 |
| Remains of amino acids (n.o) | 199 | 207 | 209 | 169 |
Micellar structure of caseins
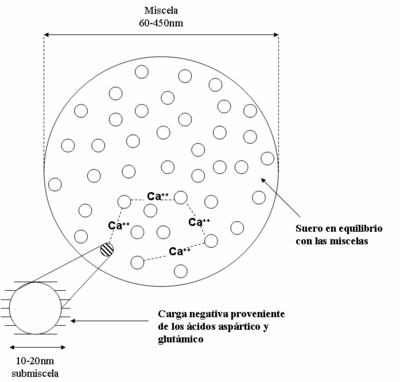
Caseins interact with each other forming a colloidal dispersion that consists of spherical particles called micelles (see Figure 3) with a diameter that usually varies between 60 and 450 nm, with an average of 130 nm. Despite the abundant scientific literature on the possible structure of a micelle, there is no consensus on the subject.

Various physicochemical models of micelle organization have been proposed, in which they are in turn made up of subunits (submicelles), with a diameter of between 10 and 20 nm (see Figure 4). In such models, the subunits are considered to be linked together thanks to calcium ions. It is suggested that calcium phosphate binds to the NH2- groups of lysine; calcium interacts with the ionized carboxyl group (COO-). Submicelles are formed from the constant interaction between α, β and κ caseins. It is necessary to highlight the function of κ-casein to stabilize the micelles, especially against the precipitation of the other protein fractions by the action of calcium or enzymes. In all of these it is established that the hydrophobic units between the protein molecules They ensure the stability of the micelle.
Uses and applications
In addition to being used directly as an adhesive in the production of food products (dairy and meat products, breads and pastry products, etc.), casein is used in the production of non-food products: glues and paints, protective coatings, plastics (see table 3).
Its technological uses are the clarification of wines or as an ingredient in molecular biology and microbiology preparations (enriched media for microbial cultivation).
In special nutrition, casein is used to prepare medical preparations and protein concentrates intended for feeding athletes, especially after their training. Thus, it has been observed that the digestion of caseins is slower than that of soluble lactoproteins (also called seroproteins) and, therefore, more appropriate to repair the anabolism of amino acids during the period that follows a meal.
| Output | Property | Implementation |
|---|---|---|
| Wrap |
|
|
| Adhesive |
|
|
| Plastic |
|
|
| Surfing |
|
|
Contenido relacionado
Dissociative drug
Lymph node
Proprioception