Caffeine
Caffeine is an antioxidant alkaloid of the xanthine group, a white crystalline solid with a bitter taste, which acts as a psychoactive drug, stimulant of the central nervous system, due to its non-selective antagonist action. of adenosine receptors. Caffeine was discovered in 1819 by the German chemist Friedrich Ferdinand Runge: it was he who coined the term Kaffein, a chemical compound present in coffee, a term that would later become Spanish. like caffeine. Caffeine also receives other names (guaranine, theine, mateine) related to the plants from which it can be extracted and because it contains other substances that appear in these cases. The so-called guaranine in guarana, and the theine in tea, are actually the same caffeine molecule, a fact that has been confirmed in laboratory analysis. These plants contain some additional alkaloids such as the cardiac stimulants theophylline and theobromine and often other chemical compounds such as polyphenols, which can form insoluble complexes with caffeine.
It is consumed by humans mainly in infusions extracted from the fruit of the coffee plant and the leaves of the tea bush, as well as various beverages and foods that contain products derived from the kola nut. Other sources include yerba mate, guayusa, the fruit of guarana, and Yaupon holly.
In humans, caffeine is a central nervous system stimulant that produces a temporary effect of restoring alertness and eliminating drowsiness. Beverages that contain caffeine, such as coffee, tea, mate, some soft drinks (especially cola), and energy drinks are very popular. Caffeine is the most widely consumed psychoactive substance in the world. In North America, 90% of adults consume caffeine every day. In the United States, the Food and Drug Administration refers to caffeine as a "Generally Recognized As Safe" food substance. (Generally Recognized As Safe) used for multiple purposes".
Caffeine has diuretic properties, if administered in sufficient doses to individuals who have no tolerance for it. Regular consumers, however, develop a strong tolerance to this effect, and studies have generally failed to substantiate the general belief that regular consumption of caffeinated beverages significantly contributes to dehydration.
Presence in nature and processed products
Caffeine is found in many plant species, where it acts as a natural pesticide. According to certain studies, the high levels of caffeine present in young plants that are still developing foliage but lack mechanical protection manage to paralyze and kill certain insects that feed on the plant. High levels of caffeine have also been found in the soils around the shoots of sprouted coffee beans. It follows from this that caffeine has a natural function not only as a natural pesticide but also as a germination-inhibiting substance of other nearby coffee beans, thus giving growing plants a better chance of survival.
The most commonly used sources of caffeine are coffee, tea, and to a lesser extent cocoa. Other less frequently used sources of caffeine include the yerba mate, guarana, and yaupon plants, which are sometimes used in the preparation of herbal teas and energy drinks.
Two of the alternative names for caffeine, mateine and guaranine, are derived from the names of these plants. Some yerba mate enthusiasts claim that mateine is actually a stereoisomer of caffeine, making it a completely different substance. This is not true since caffeine is a non-chiral molecule and therefore has no enantiomers, nor does it have other stereoisomers. The disparity in experience and effects between the various natural sources of caffeine could be due to the fact that Plant sources of caffeine also contain widely variable mixtures of other xanthine alkaloids, including the cardiac stimulants theophylline and theobromine, as well as other substances that together with caffeine can form insoluble complexes, such as polyphenols.
One of the primary sources of caffeine throughout the world is the coffee bean (the seed of the coffee plant), from which the coffee beverage is made. The caffeine content in coffee varies widely depending on the type of coffee bean and the preparation method used; even the grains found in the same bush can present variations in concentration. In general, a serving of coffee ranges from 40 milligrams for a 30-milliliter espresso of the Arabica variety, up to about 100 milligrams for a cup (120 milliliters) of coffee. Roasted coffee generally has less caffeine than light coffee because the roasting process reduces the caffeine content of the bean. Coffee of the arabica variety usually contains less caffeine than the robust variety. Coffee also contains trace amounts of theophylline, but not theobromine.
Tea is another common source of caffeine. Although tea contains more caffeine than coffee, a typical serving contains much less as tea is typically brewed much more dilute. In addition to the higher or lower concentration of the brew, growing conditions, processing techniques, and other variables also affect caffeine content. Certain types of tea may contain more caffeine than others. Tea contains small amounts of theobromine and slightly higher levels of theophylline than coffee. Brewing and other factors have a significant impact on tea, and color is a very poor indicator of caffeine content. Some varieties, such as Japanese pale green tea gyokuro, for example, contain more caffeine than darker ones such as lapsang souchong, which contains very little.
Caffeine is also a common ingredient in many non-alcoholic beverages (especially carbonated beverages), such as colas originally made from the kola nut. These drinks typically contain between 10 and 50 milligrams of caffeine per serving. In contrast, energy drinks can contain more than 80 milligrams of caffeine per serving. The caffeine in these drinks is present in the ingredients used in them, or is added. Guarana, a primary ingredient in energy drinks, contains large amounts of caffeine with small amounts of theophylline and theobromine along with a natural excipient that produces a slow release of these substances.
In recent years some manufacturers have begun to add caffeine to hygiene products such as shampoo and soap, ensuring that the caffeine can be absorbed through the skin. The effectiveness of such products, however, has not been proven, and it is likely that they have little effect on the central nervous system since caffeine is not readily absorbed through the skin.
| Output | Size of ration | Caffeine per ration (mg) | Coffee per litre (mg) |
|---|---|---|---|
| Caffeine tablets (normal) | 1 pill | 100 | - |
| Caffeine tablets (extra strong) | 1 pill | 200 | - |
| Excedrin pills | 1 pill | 65 | - |
| Chocolate (45 % cocoa) | 1 bar (43 g) | 31 | - |
| Milk chocolate (11 % cocoa) | 1 bar (43 g) | 10 | - |
| Coffee (domestic coffee maker) | 207 mL | 80-135 | 386-652 |
| Coffee (filter coffee maker) | 207 mL | 115-175 | 555-845 |
| Coffee, decaf | 207 mL | 5-15 | 24-72 |
| Coffee, espresso | 44–60 mL | 100 | 1691-2254 |
| Black tea | 177 mL | 50 | 282 |
| Green tea | 177 mL | 30 | 169 |
| Coca-Cola | 355 mL | 34 | 96 |
| Mountain Dew | 355 mL | 54.5 | 154 |
| Jolt Cola | 695 mL | 280 | 402 |
| Red Bull | 250 mL | 80 | 320 |
| Yerba mate, Guayakí | 6g (loose leaves) | 85 | approx. 358 |
Some manufacturers market caffeine pills, claiming that pharmaceutical-grade caffeine promotes mental alertness. These effects have been suggested by studies showing that the use of caffeine (whether in pill form or not) causes a decrease in feelings of fatigue and an increase in attention span. These pills are commonly used by students preparing for their exams and by people who work or drive long hours.
Discovery and synthesis
In 1819, the German chemist Friedlieb Ferdinand Runge isolated a relatively pure caffeine for the first time. He did this work at the request of Johann Wolfgang von Goethe. Pierre Joseph Pelletier and Pierre Jean Robiquet described it in 1821. M. Oudry isolated theine from tea in 1827, and Gerardus Mulder and Jobst demonstrated in 1838 that it was the same. substance than caffeine. The structure of caffeine was elucidated towards the end of the 19th century by Hermann Emil Fischer who was the first to achieve its total synthesis. Fischer was moreover awarded the Nobel Prize in chemistry in 1902 in part for this work.
The aromatic character of caffeine is due to the fact that the nitrogen atoms are practically in the same plane (in the sp² hybridization orbital). Caffeine is generally not produced by synthesis because it is available in large quantities as a byproduct of decaffeination.
Caffeine can be synthesized from dimethylurea and malonic acid.
History
Humans have consumed caffeine since the Stone Age. Ancient peoples discovered that chewing the bark and leaves of certain plants had the effect of relieving fatigue, stimulating alertness, and elevating mood. Only much later was it discovered that the effect of caffeine was increased by soaking such plants in hot water.[citation needed] Many cultures have legends attributing the discovery of such plants to people who would have lived many thousands of years before, as in the case of the Chinese emperor Shennong.
According to a popular Chinese legend, Shennong would have reigned around 3000 BC, he accidentally discovered that when some leaves were dropped into boiling water, the result was an aromatic and restorative drink. Shennong is also mentioned in Lu Yu's Cha Jing, the famous first monographic work on tea. The history of coffee has been recorded since the IX century. At that time, coffee beans were only available in the plant's natural habitat, Ethiopia. A popular legend attributes its discovery to a goat farmer named Kaldi, who apparently observed that the goats became euphoric and lost sleep at night after having grazed next to the coffee bushes and, having tasted the fruits that goats had been eating, he experienced the same vitality. The first literary mention of coffee could be a reference to Bunchum in the works of the Persian physician of the IX century Al-Razi. In 1587, Malaye Jaziri compiled a work tracing the history and legal controversies of coffee, entitled Undat al safwa fi hill al-qahwa. In this work, Jaziri recorded that a Sheikh, Jamal-al-Din al-Dhabhani, Mufti of Aden, first adopted the use of coffee in 1454, and that in the XV Sufis in Yemen routinely used coffee to stay awake during prayers.
Near the end of the 16th century, the use of coffee was recorded by a European residing in Egypt, and around In this period its general use in the Near East is introduced. The appreciation of coffee as a beverage in Europe, where it was initially known as "Arabic wine", dates back to the 17th century. During this period "coffee houses" were established, with the first opening in Constantinople and Venice.
After Italy, it spread through Spain, and from there it went to France. Its heyday begins with the Siege of Vienna, in 1683. Marco d'Aviano, a Capuchin father, used the coffee abandoned by the Turks when they withdrew from it, made an infusion and mixed it with cream.
Coffee was considered a medicine and an aphrodisiac, earning it the label "diabolical" by some members of the Catholic Church. The anecdote (of whose veracity there are doubts) that Clemente VIII starred in, whose advisers wanted to ban coffee definitively, is well known. The pope, before making such a decision, wanted to taste the drink and declared "Questa bevanda del diavolo è così buona... che dovremmo cercare di ingannarlo e battezzarlo" (This devil's drink is so good...we should see how to trick him into naming it). After papal approval, its consumption increased. Another version indicates that the pope's personal physician, Andrea Cesalpino, prescribed coffee to alleviate the emotional disorders suffered by the pontiff (probably anxiety or bipolar disorder). Cesalpino was also a botanist and was the first in Europe to describe the coffee plant in detail, including the effects of caffeine.
In Britain, the first coffee houses were opened in London in 1652, in St Michael's Alley, Cornhill. They soon became popular throughout Eastern Europe as well, and played a significant role in social relations during the 17th and 18th centuries.
As mentioned, both the kola nut, the coffee fruit and the tea leaf all have ancient origins. It is chewed in various West African cultures, individually or in social gatherings, to restore vitality and allay hunger pangs. In 1911, cola became the focus of one of the first documented health fears, when the United States government seized 40 casks and 20 barrels of Coca-Cola syrup in Chattanooga, Tennessee, alleging that the caffeine in his drink was "harmful to health." On March 13, 1911, the government brought the case of The United States v. Forty Casks and 20 Barrels of Coca-Cola, hoping to force Coca-Cola to eliminate caffeine from its formula on the grounds such as that the excessive use of Coca-Cola in a girls' college led to "nocturnal debauchery, breaking school rules and feminine manners, and even fostering immorality." Although the judge ruled in favor of Coca-Cola, two bills were introduced in the House of Representatives in 1912 to amend the Pure Food and Drug Act, adding caffeine to the list of substances." habit-forming” and “harmful” that had to be listed on the product label.
Chemical properties
Caffeine is an alkaloid of the methylxanthine family, whose metabolites include theophylline and theobromine compounds, with a similar chemical structure and similar effects (although less intense at the same doses). In its pure state it is a very bitter white powder. It was discovered in 1819 by Runge and described in 1821 by Pelletier and Robiquet.
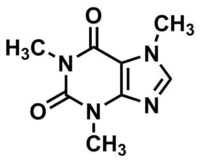
Its chemical formula is C8H10N4O2, its systematic name is 1, 3,7-trimethylxanthine or 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione and its structure can be seen in the included diagrams.
A cup of coffee contains 80 (instant) to 125 (filtered) mg of caffeine. Decaffeinated coffee, in Spain, must contain an amount of caffeine not exceeding 0.3%. Caffeine is also available in stim pills of up to 800mg.
Pharmacology
Global caffeine consumption was estimated at 120,000 tons per year, making it the most popular psychoactive substance. Caffeine is a central nervous system and metabolic stimulant, and is used both recreationally and medically to reduce physical fatigue and restore mental alertness in cases of unusual weakness or lethargy. Caffeine and other methylxanthine derivatives are also used in newborns to treat apnea and to correct irregular heartbeats. Caffeine activates the central nervous system at higher levels, causing increased alertness and wakefulness, a faster and clearer flow of thought, increased attention, and improved body coordination. Then it acts at the level of the spinal cord when it is in high doses. Once inside the body, it has a complex chemistry that works through different mechanisms of action that are described below.
Metabolism and half-life
Caffeine from coffee and other infusions is absorbed by the stomach and small intestine within 45 minutes of ingestion and is then distributed throughout all tissues of the body. The effects of caffeine begin to be felt in 10 minutes Its elimination follows first-order kinetics. Caffeine can also be ingested rectally, as demonstrated by the prescription of ergotamine tartrate and caffeine suppositories (for migraine relief), and chlorobutanol and caffeine (for the treatment of hyperemesis).
The half-life of caffeine—that is, the time required for the body to eliminate half of the initial total amount of caffeine—varies widely among individuals according to factors such as age, liver function, pregnancy, some concurrent drugs and the level of enzymes in the liver necessary for the metabolism of caffeine. In healthy adults, the half-life of caffeine is about 4-9 hours. In women taking oral contraceptives, the half-life is 5-10 hours, and in pregnant women the half-life is approximately 9-11 hours. Caffeine can accumulate in individuals with severe liver disease, increasing its half-life even up to 96 hours. In infants and children the half-life may be longer than in adults; the half-life in a newborn can be up to 30 hours. Other factors such as smoking can shorten the half-life of caffeine. Fluvoxamine reduces caffeine elimination by 91.3%, and prolongs its half-life by 11.4 times normal (this is 4 times normal). 9 hours to 56 hours).
Caffeine is metabolized in the liver by the cytochrome P450 oxidase enzyme system (specifically, the 1A2 isoenzyme) into three dimethylxanthine metabolic products, each of which has its own effects on the body, which are:
- Paraxantin (84 %): Increases lipolyis by inducing increased levels of glycerol and free fatty acids in blood plasma.
- Theobromine (12 %): Dilates blood vessels and increases urine volume. Theobromine is also the main alkaloid in the cocoa.
- Theophylline (4 %): Relax the smooth muscle of the bronchus and is thus used for the treatment of asthma. However, the therapeutic dose of theophylline is of a greater multiple than that obtained by the metabolism of caffeine.
Each of these metabolites is then metabolized and excreted in the urine.
Mechanism of action
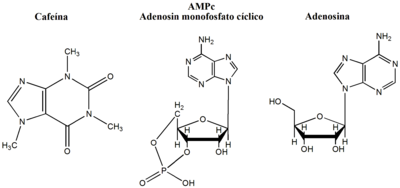
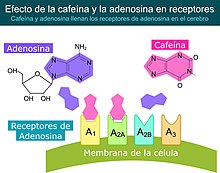
Caffeine's primary mode of action is as an antagonist of adenosine receptors found on brain cells.
Caffeine easily crosses the blood-brain barrier that separates the blood vessels from the brain. Once in the brain, the primary mode of action is as a non-selective adenosine receptor antagonist. The caffeine molecule is structurally similar to adenosine and therefore binds to adenosine receptors on the surface of cells. cells without activating them (an "antagonist" mechanism of action). So caffeine acts as a competitive inhibitor.
Adenosine is found almost everywhere in the body, because it plays a key role in ATP-related energy metabolism, but in the brain, adenosine plays special roles. There is evidence indicating that brain adenosine concentrations are increased by various types of metabolic stress, among which we cite: hypoxia and ischemia. Evidence also indicates that brain adenosine acts to protect the brain by suppressing neuronal activity and also by increasing blood flow through the A2A and A2B receptors. > located in vascular smooth muscle. By counteracting adenosine, caffeine reduces resting cerebral blood flow by 22 to 30%. Caffeine also has a general disinhibitory effect on neuronal activity. However, it has not been shown how these effects cause an increase in wakefulness and alertness.
Adenosine is released into the brain by a complex mechanism. There is evidence indicating that adenosine functions as a neurotransmitter released into synaptic gaps in some cases, however stress-related increases in adenosine appear to be produced mainly through the extracellular metabolism of ATP. Adenosine is certainly not the primary neurotransmitter of any group of neurons, but it is released along with other neurotransmitters by some types of neurons. Unlike many neurotransmitters, it appears that adenosine is not stored in vesicles that are voltage-dependent, so the possibility of this mechanism has not been completely ruled out. Several classes of adenosine receptors have been described, each with different anatomical locations. A1 receptors are widely distributed and act by inhibiting calcium absorption. A2A receptors are densely concentrated in the basal ganglia, an area that plays a critical role in behavioral control, but they can also be found in other parts of the brain but at lower densities. There is evidence that A 2A receptors interact with the dopaminergic system, which is involved in wakefulness and reward. A2A receptors can also be found on arterial walls and on the cell membranes of blood cells.
Beyond its neuroprotective effects, there are reasons to believe that adenosine may be more specifically involved in the control of sleep-wake cycles. Robert McCarley and colleagues opine that adenosine accumulation may be a primary cause of the sensation of sleepiness that follows prolonged mental activity, and that the effects may be mediated both by inhibition of wake-promoting neurons via A receptors 1, and by activation of sleep-promoting neurons mediated by indirect effects at A2A receptors. Recent studies have provided additional evidence on the importance of A receptors 2A, but not for A1.
Some of the side effects of caffeine are probably caused by effects unrelated to adenosine. Like other methylated xanthines, caffeine is also a:
- Competitive and non-selective inhibitor of phosphodiesterase which increases intracellular cAMP, activates PKA, and inhibits TNF-alfa and synthesis of leucotriene, reduces inflammation and innate immune system
- Non-selective adenosine receptor antagonist
Phosphodiesterase inhibitors inhibit cAMP-phosphodiesterase (cAMP-PDE) enzymes, which convert cyclic AMP into its non-cyclic form within cells, thus allowing cAMP production within cells. cells. Cyclic AMP is involved in the activation of protein kinase A (PKA) which in turn initiates the phosphorylation of specific enzymes involved in glucose synthesis. By blocking its breakdown, caffeine intensifies and prolongs the effects of epinephrine and epinephrine-type drugs such as amphetamines, methamphetamines, or methylphenidates. In turn, high cAMP concentrations in parietal cells cause an increase in cAMP-dependent protein kinase A activation, which in turn increases proton pump activation, specifically H+/K+ ATPase, with the effect last, an increase in the secretion of acidic gastric juices.
Cyclic AMP also increases the activity of the If current, which in turn directly increases heart rate. Caffeine is also a structural analogue of strychnine and like it (although much less potent) is a competitive antagonist of glycine ionotropic receptors.
Caffeine metabolites also contribute to its effects. Paraxanthin is responsible for increasing the process of lipolysis, which releases glycerol and fatty acids into the bloodstream to be used as energy by the muscles. Theobromine is a vasodilator that increases the amount of oxygen and nutrient flow to the brain and muscles. Theophylline acts as a smooth muscle relaxant that mainly affects the bronchioles and also acts as a chronotropic and inotropic substance increasing heart rate and efficiency.
Drug Interactions
- The caffeine concentration in the body can decrease with the induction of its metabolism at the level of CYP1A2 that produce it among others: tobacco smoke, carbonized meat or a decrease in the body fat index. In this way, smokers who consume coffee and leave the smoking habit can double the plasma concentrations of caffeine by showing symptoms of poisoning.
- Caffeine concentration can also be increased in the body if its metabolism is inhibited, through physiological/pathological processes such as the end of pregnancy, liver disease or obesity. There are also substances such as alcohol, grapefruit juice or drugs (ketoconazole, fluoxetin, paroxetine...) that produce this effect.
- Caffeine can also increase the absorption of paracetamol, acetylsalicylic acid and ergotamine thus increasing its bioavailability. On the other hand, caffeine can increase the concentrations of teofiline and chlorzapine by interacting in its elimination process.
Caffeine intake in psychiatric patients
Increased public education on potential health problems associated with caffeine use is suggested, and more control of caffeine in the psychiatric setting is recommended. The massive use of the use of caffeine in psychiatric patients can lead to the exacerbation of the symptoms of these patients.
There are also pharmacokinetic and pharmacodynamic interactions between high doses of caffeine and antipsychotic drugs. Several psychoactive drugs interact with caffeine in vitro, forming insoluble precipitates, which may occur in vivo and prevent the absorption of both caffeine and antipsychotics and probably increase psychotic symptoms as the dose of drug absorbed is lower than prescribed.
Competitive antagonism between caffeine and some benzodiazepines such as diazepam in ligation areas of the central nervous system has also been cited. They suggest that caffeine blocks these areas in vivo for high-dose benzodiazepines, resulting in a reversal of the tranquilizing effects of diazepam, causing anxiety instead of an anxiolytic effect. As well as the use of caffeine with hypnotic sedatives antagonizing its action.
Health effects
Consumption in very large quantities can cause poisoning. Its symptoms are: insomnia, nervousness, excitement, reddish face, increased diuresis and gastrointestinal problems. In some people, symptoms appear when very small amounts are consumed, on the order of 250 mg per day. More than one gram a day can cause involuntary muscle contractions known as fasciculations, delusions, cardiac arrhythmia, and psychomotor agitations. The symptoms of caffeine intoxication are similar to those of panic and generalized anxiety, with effects typical of dissociative drugs such as depersonalization. The estimated LD50 of caffeine is 10 g, which is equivalent to an average of 100 cups of coffee.
Several scientific publications and regulatory entities, such as the European Food Safety Authority (EFSA), warn that the increasing consumption of beverages and other products, with considerable concentrations of caffeine both in sports and in other settings, may have negative effects on health, particularly among children and young people.
Caffeine is a vasoconstrictor, which is why it is usually used together with paracetamol for rapid relief of headaches. However, for this reason it is contraindicated in cases of high blood pressure.
Another effect of caffeine in the body is on spermatozoa: at low doses (1 glass a day), it has the ability to activate their mobility, increasing the probability of success in natural fertilization, but its consumption in higher doses It is usually related to male infertility problems.
Effect on children
In healthy children, caffeine produces modest and innocuous effects. The Canadian Health Association recommends that children under 12 years of age should not be given more than 2.5 milligrams of caffeine per kilogram of body weight.
Studies show that caffeine can be used to treat children with attention deficit disorder. Research found that 200-300 mg of caffeine produced a similar effect to ritalin, without its side effects.
Caffeine consumption in sports
Caffeine was studied for its possible benefit in sports activities that require endurance capacity. Early studies demonstrated notable improvements in cyclists' endurance when compared to those obtained when a placebo drink was consumed.
Recent studies indicate that caffeine temporarily increases strength in sports such as weightlifting.
Controversies over "mathene"
According to a study carried out in 2011 by a team from the Faculty of Engineering of the FCEIA led by T.A., it is possible to affirm that "mateína" does not exist as such. This team concluded that the natural stimulant present in yerba mate is actually caffeine.
Contenido relacionado
Health
Sulfur
Copper