Butane
Butane, also called n-butane, is a saturated, paraffinic or aliphatic, flammable, gaseous hydrocarbon that liquefies at atmospheric pressure at -0.5 ° C, formed by four carbon atoms and ten hydrogen, whose chemical formula is C4H10. An isomer of this can also be called by the same name gas: isobutane or methylpropane.
Commercial butane is a liquefied gas, obtained by distillation of petroleum, composed mainly of normal butane (60%), propane (9%), isobutane (30%) and ethane (1%).
As it is a colorless and odorless gas, an odorant (usually a mercaptan) is added during its preparation, which gives it an unpleasant odor. This allows it to be detected in case of leaks, because being very volatile it can accumulate in a room and cause an explosion.
Different methods are used to extinguish a fire caused by butane gas, such as carbon dioxide (CO2), dry chemical or water fog.
Applications
The main application of butane gas (C4H10) is as fuel in homes for cooking, heating water, in stoves and in pocket gas lighters.
In Spain
In Spain, butane gas is transported in the typical bottle or butane bottle, which is a cylindrical container, with steel walls, usually orange in color (also called "butane color" for this reason)., and containing 12.5 kg of butane, most of which is in a liquid state, under pressure. There are also new lighter butane cylinders, made from stainless steel instead of cast iron.
Its regulation appears in Royal Decree 1085/1992 of September 11, which approves the Regulation of the activity of distribution of Liquefied Petroleum Gases. In its article 22, it determines the obligations of the holders of said supply contracts. Among them is the inspection of the installation every five years by a legally authorized installation company. Similar measures have been taken in other countries.
In its pure state, butane is odorless; however, to make it more easily detectable in the case of leaks, mercaptan sulfide is added as an odorant compound that makes it perceptible to smell before the butane-air mixture can be explosive.
Physical-chemical properties
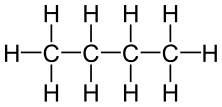
Its structural formula is:
- Color: colorless.
- Olor: inolent substance when it is pure, so it is added another substance (usually methyl mercaptano) of characteristic odor in order to avoid accidents.
- Relative density of steam (air=1): 2.1.
- Density in liquid state (at 16 °C): 0,582 g/cm3
- Solubility in water: 3.25 ml/100 ml at 20 °C.
- Evaporation point: -1 °C.
- Fusion point: -138 °C.
- Molar mass: 58 g/mol.
- Higher heat power: 49 608 kJ/kg approx.
- Combustion detail: -2 880 kJ/mol.
Health Effects
Butane is not toxic, although being heavier than air tends to displace it and can cause death by suffocation by preventing air from reaching the lungs and oxygenating the blood.
Butane inhalation can cause euphoria, drowsiness, unconsciousness, suffocation, heart rhythm disturbances, blood pressure fluctuations, temporary memory loss, when directly abusing a pressurized container, and may result in death by asphyxia and ventricular fibrillation. It enters the bloodstream and, in seconds, produces intoxication.
Butane is the most commonly misused volatile substance in the UK, and was the cause of 52% of solvent-related deaths in 2000. By spraying butane directly down the throat, the stream of liquid can quickly cool down to −20 °C by expansion, causing prolonged laryngospasm. A "sudden death syndrome", first described in 1970, is the most common single cause of solvent-related death, resulting in 55% of known fatal cases.
A small amount of nitrogen dioxide, a toxic gas, results from the burning of butane gas, along with any combustion in Earth's atmosphere, and poses a hazard to human health from butane heaters and stoves.
Contenido relacionado
Noble gases
Hasio
Benzene