Bromine
Bromine is a chemical element with atomic number 35 located in the halogen group (group VII A) of the periodic table of elements. Its symbol is Br.
Bromine is a dense, volatile, red liquid at room temperature. Its reactivity is intermediate between chlorine and iodine. In the liquid state it is dangerous for human tissue and its vapors.
Elemental bromine is highly reactive and is therefore not found free in nature, but in colorless crystalline halide mineral salts, analogous to table salt. Although it is quite rare in the Earth's crust, the high solubility of the bromide ion (Br-) has caused its accumulation in the oceans. Commercially, the element is readily extracted from brine evaporation ponds, most notably in the United States, Israel, and China. The mass of bromine in the oceans is about one three hundredth that of chlorine.
Under standard conditions of pressure and temperature it is a liquid; the only other element that is liquid under these conditions is mercury. At high temperatures, organobromine compounds readily dissociate to free bromine atoms, a process that stops free radical chemical chain reactions. This effect makes organobromine compounds useful as fire retardants, and more than half of the bromine produced worldwide each year is used for this purpose. The same property causes ultraviolet sunlight to split volatile organobromine compounds in the atmosphere into free bromine atoms, causing ozone depletion. As a result, many organobrominated compounds - such as the pesticide methyl bromide - have ceased to be used. Bromine compounds continue to be used in well-drilling fluids, photographic film, and as an intermediate in the manufacture of organic chemicals.
Bromide salts in large amounts are toxic by the action of soluble bromide ions, causing bromism. However, a clear biological role for the bromide ion and hypobromous acid has recently been elucidated, and bromine now appears to be an essential trace element in humans. The role of biological organobromine compounds in marine life such as algae has been known for much longer. As a pharmaceutical, the simple bromide ion (Br-) has inhibitory effects on the central nervous system, and bromide salts were once an important medical sedative, before being replaced by shorter-acting drugs. They retain specialized uses as antiepileptics.
History
Bromine (from the Greek bromos, meaning "stink" or pestilence) was discovered in 1826 by Antoine-Jérôme Balard. and independently by Carl Jacob Löwig in 1825 and 1826, respectively, but it was not produced in significant numbers until 1860.
Löwig isolated bromine from a mineral spring in his hometown of Bad Kreuznach in 1825. Löwig used a chlorine-saturated solution of the mineral salt and extracted the bromine with diethyl ether. Upon evaporation of the ether, a brown liquid remained. With this liquid as a sample of his work he applied for a position in Leopold Gmelin's laboratory in Heidelberg. The publication of the results was delayed and Balard published his results first.
Balard found bromine chemicals in algae ash from the Montpellier salt marshes. The algae were used to produce iodine, but they also contained bromine. Balard distilled the bromine from a chlorine-saturated solution of algae ash. The properties of the resulting substance were intermediate between those of chlorine and iodine, so he tried to prove that the substance was iodine monochloride (ICl), but after failing in his attempt he was sure he had found a new element and named it mururo, derived from the Latin word muria. ("brine").
After French chemists Louis Nicolas Vauquelin, Louis Jacques Thénard and Joseph-Louis Gay-Lussac approved the young pharmacist Balard's experiments, the results were presented at a conference at the Académie des Sciences and published in Annales de Chimie et Physique. In his publication, Balard stated that he had changed his name from muride to brôme at the suggestion of M. Anglada. The name brôme (bromo) derives from the Greek βρῶμος (brômos, "stink"). Other sources state that the French chemist and physicist Joseph-Louis Gay-Lussac suggested the name brôme due to the characteristic odor of the vapors.}} Bromine was not produced in large quantities until 1858, when the discovery of salt deposits at Stassfurt allowed its production as a by-product of potash.
Apart from some minor medical applications, the first commercial use was the daguerreotype. In 1840, bromine was found to have some advantages over the iodine vapor previously used to create the light-sensitive silver halide layer in daguerreotypes.
Abundance and obtaining
Most of the bromine is found in the sea in the form of bromide, Br-. In the sea it presents a concentration of about 65 µg/g.
Molecular bromine, Br2 is obtained from brines, by oxidizing bromide with chlorine, once obtained:
- 2Br - + Cl2 → Br2 + 2Cl-
It is necessary to use a distillation process to separate it from the Cl2.
Approximately 500 million kilograms of bromine are produced in the world per year (2001). The United States and Israel are the main producers. The waters of the Dead Sea and the Stassfurt mines are rich in potassium bromide.
Compounds
It can present different oxidation states. The most common are -1 (most common), +1 (with chlorine), +3 (with fluorine), and +5 (with oxygen).
- The oxidation condition +1 is unstable, but very oxidant from the kinetic point of view, in aqueous dissolution and disproportionate to the oxidation states -1 and +5. For example, hypobromite ion, BrO- (only stable at low temperatures 0 °C).
- The oxidation condition +3 is unstable in aqueous dissolution and disproportionates the oxidation states +1 and +5. For example, ion bromito, BrO2-, or bromosus acid, HBrO2 (very unstable).
- The oxidation condition +5 is thermodynamically stable against disproportion in aqueous dissolution. For example, the Joke ion, BrO3-. Joke is a strong oxidant (such as permanganate) more oxidant than chlorate and kineticly more reactive. It is also a carcinogen (very strong suspects).
- The perbromato ion, BrO4-, with a oxidation condition +7, it is reduced with relative ease and prepared with difficulty: using elemental fluoride or electrolytic methods, is a very strong 1,8 oxidant although somewhat slow from the kinetic point.
BrO3F (perbromyl fluoride) is a new agent much more unstable than its chlorinated analog and so reactive it destroys even Teflon. It is also a Lewis acid unlike its chlorinated homologue perchloryl fluoride, forming a BrO3F2(-1) complex analogous to XeO3F2. When reacting with Lewis acids the bromine is reduced to +5 releasing oxygen, the chlorinated analog does not react with antimony pentafluoride SbF5.
- Bromine also forms compounds with other halogens (interhalgens). For example, BrF5, BrF3IBr, etc.
BrF5 is a liquid that reacts explosively with almost all substances, very similar in reactivity to ClF3, capable of burning substances used as fire extinguishers, water, glass, oxides, halides and a wide variety of inorganic substances react, organic substances react explosively.
- There are many compounds in which the bromine has a state of oxidation -1, calling these bromurs.
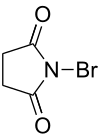
Brominated organic compounds can be easily obtained, for example, by radical bromination with molecular bromine and in the presence of light or using N-bromosuccinimide, or by addition or substitution reactions. The organic compound methyl bromide, CH3Br, is used as a pesticide, but it affects the ozone layer. It has been determined that bromine atoms are more effective than chlorine atoms in the ozone layer destruction mechanisms, however bromine atoms are in less quantity.
Hydrogen bromide, HBr, is obtained by direct reaction of bromine with molecular hydrogen or as a by-product of bromination processes of organic compounds.At 400º it attacks glass.It is very acidic. From this, different bromides can be obtained, for example:
- HBr + NaOH → NaBr + H2O
It is much more unstable than its chlorinated analog and is reducing.
Nitric acid oxidizes bromides in the presence of nitrites vigorously.
Bromine in aqueous solution can disproportionate:
- Br2 + OH- → Br- + BrOH
But the reaction does not take place in an acid medium.
The ion Br2+ can also be obtained by oxidation.
Hydrogen bromide
The simplest bromine compound is hydrogen bromide, HBr. It is mainly used in the production of inorganic bromides and alkyl bromides, and as a catalyst for many reactions in organic chemistry. Industrially, it is mainly produced by the reaction of hydrogen gas with bromine gas at 200-400 °C with a platinum catalyst. However, bromine reduction with red phosphorus is a more practical way to produce hydrogen bromide in the laboratory:
- 2 P + 6 H2O + 3 Br2 → 6 HBr + 2 H3PO3
- H3PO3 + H2O + Br2 → 2 HBr + H3PO4
At room temperature, hydrogen bromide is a colorless gas, like all hydrogen halides except hydrogen fluoride, since hydrogen cannot form strong hydrogen bonds with the bromine atom, which is large and few electronegative; however, in solid crystalline hydrogen bromide there are weak hydrogen bonds at low temperatures, similar to those in the structure of hydrogen fluoride, before disorder begins to prevail as the temperature is raised. Aqueous hydrogen bromide is known as hydrobromic acid, which is a strong acid (P < 9) because the hydrogen bonds with bromine are too weak to inhibit dissociation. The HBr/H2O system also includes many HBr-nH2O hydrates for n = 1, 2, 3, 4 and 6, which are essentially salts of bromine and hydronium anions. cation. Hydrobromic acid forms an azeotrope with a boiling point of 124.3 °C at 47.63 g HBr per 100 g solution; therefore, hydrobromic acid cannot be concentrated beyond this point by distillation.
Other binary bromides
Almost all the elements on the periodic table form binary bromides. The exceptions are decidedly in the minority and derive in each case from one of three causes: extreme inertia and reluctance to participate in chemical reactions (the noble gases, with the exception of xenon in the highly unstable XeBr 2); extreme nuclear instability making chemical research difficult before decay and transmutation (many of the heavier elements beyond bismuth); and that it has an electronegativity higher than that of bromine (oxygen, nitrogen, fluorine and chlorine), so that the resulting binary compounds are not formally bromides but oxides, nitrides, fluorides or chlorides of bromine. However, nitrogen tribromide is called a bromide, since it is analogous to the other nitrogen trihalides.
Bromine halides
Halogens form many binary compounds, diamagnetic interhalogens with stoichiometries XY, XY3, XY5, and XY7 (where X is heavier than Y), and bromine is no exception. Bromine forms a monofluoride and a monochloride, as well as a trifluoride and a pentafluoride. Some cationic and anionic derivatives are also characterized, such as BrF−
2, BrCl−
2, BrF+
2 , and BrF+
6. In addition to these, some pseudohalides are also known, such as cyanogen bromide (BrCN), bromine thiocyanate (BrSCN), and bromine azide (BrN3).
Polybromine Compounds
Although dibromine is a strong oxidizing agent with a high first ionization energy, very strong oxidants such as peroxydisulfuryl fluoride can oxidize it to the cherry red +2Br cation.
Bromine oxides and oxoacids
The oxides of bromine are not as well characterized as the oxides of chlorine or the oxides of iodine, since they are all quite unstable: it was once thought that they could not exist at all. Dibromine monoxide is a dark brown solid that, while reasonably stable at -60°C, decomposes at its melting point of -17.5°C; it is useful in bromination reactions and can be obtained from the low-temperature decomposition of bromine dioxide in a vacuum. Oxidizes iodine to iodine pentoxide and benzene to 1,4-benzoquinone; in alkaline solutions, it gives the hypobromite anion.
Biological paper
Bromine is found at trace levels in humans. It is considered an essential chemical element, although the functions it performs are not exactly known. Some of its compounds have been used in the treatment of epilepsy and as sedatives.
Isotopes
Two isotopes are found in nature: 79Br and 81Br, both with an abundance of about 50%.
Applications
The chemical and industrial applications of bromine are numerous and varied, highlighting organobrominated compounds, which are prepared from diatomic bromine or from hydrogen bromide (hydrobromic acid in aqueous solution).
The bromine test consists of the use of bromine water in order to detect the presence of unsaturated organic compounds.
Bromides act medically as sedatives and silver bromide is used as a fundamental element in photographic plates.
Precautions
Elemental bromine is highly toxic and from small traces (10 ppm), both through the skin and the respiratory route, it can cause immediate health problems and death in larger doses. It is very irritating to both the eyes and the throat; in contact with the skin produces painful burns. Improper handling poses a serious health risk, requiring maximum precautions for its safe management.
Contenido relacionado
Gas chromatography
Molecularity
Glass