Benzene
Benzene is an aromatic hydrocarbon with the molecular formula C6H6 (originally it and its derivatives were called aromatic compounds due to to the characteristic shape they possess). It is also known as benzol. In benzene each carbon atom occupies the vertex of a regular hexagon, apparently three of the four valencies of the carbon atoms are used to bond adjacent carbon atoms together, and the fourth valence with a hydrogen atom. According to modern theories of chemical bonding, three of the four electrons in the valence shell of the carbon atom are used directly to form typical covalent bonds (2C-C and C-H) and the fourth is shared with those of the other five. carbon atoms, obtaining what is called "the π (pi) cloud" containing the six electrons in various orbitals. Benzene is a colorless and highly flammable liquid with a sweet smell (which must be handled with great care due to its carcinogenic nature), with a relatively high boiling point.
Benzene is used in large amounts in the United States. It is on the list of the 20 chemical products with the highest production volume. Some industries use benzene as a starting point to manufacture other chemicals used in the manufacture of plastics, resins, nylon, and synthetic fibers such as Kevlar and certain polymers. Benzene is also used to make certain types of rubber, lubricants, dyes, detergents, toner for laser printers, medicines, and pesticides. Volcanoes and forest fires are natural sources of benzene. Benzene is also a natural component of crude oil and gasoline. It is also found in cigarette smoke and other organic materials that have been burned. It can be obtained by fractional distillation of coal tar.
It is usually shown, in terms of Lewis structure, as a hexagon, flat and undeformable, devoid of ring tensions (transannular), in whose vertices are the carbon atoms, with three double bonds and three single bonds in alternate positions (1=2, 3=4, 5=6; 6-1, 2-3, 4-5; or 1=2-3=4-5=6-1). This structure differed from that of Brønsted and Lowry. It should be noted that, according to the results of infrared spectrophotometry, benzene has neither single nor double bonds, but rather a resonance hybrid between the two, with an average bond distance between single and double (approximately 1.4 Å). These results agree with the prediction of the TOM (molecular orbital theory), which calculates a distribution of three fully occupied bonding orbitals. This special stability is called aromaticity and the molecules (ions or not, stable or reaction intermediates) are called aromatic.
Historical introduction
The benzene molecule was discovered by Faraday in 1825, who first isolated the compound, with the empirical formula CH, from lighting gas. It was Eilhard Mitscherlich who managed to measure its molecular mass from its vapor pressure, establishing it at 78 u, which corresponded to a molecular formula C6H6. The compound had been obtained from benzoin gum, which led to its being called benzine, and later benzene.
Initially open forms (aliphatic) were proposed for the benzene chain, with two triple bonds, however the experimental data obtained from its reactions were contradictory with these open models, since it presented an unusually low number of isomers. Thus, for example, the monobromination of the compound presented a single isomer, as was the case with nitration. On the other hand, it did not respond to the usual additions of nucleophiles to multiple bonds.
This led to various structures being proposed to understand these facts, such as Dewar's, Klaus' or Kekulé's.
However, the Kekulé structure continued to present an incompatibility with the 1,2 malformation of the molecule, since two isomers (orthobenzene isomers) would have to be formed, one of them with bromine on a double bond and the other with both benzenes over a single bond. This led Kekulé to propose that benzene alternated between two forms, in which the bonds continually changed position, so only one isomer would be detected.
Resonance of benzene
The representation of the three double bonds is due to Friedrich Kekulé, who was also the discoverer of the ring structure of said compound and the first to represent it that way.
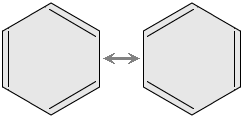
Anyway, it was the Nobel Prize in Chemistry, Linus Pauling, who managed to find the true origin of this behavior, resonance or mesomerism, in which both Kekulé structures overlap.

It is usually represented as a regular hexagon with an inscribed circle to note that the three double bonds in benzene are delocalized, dissociated, and resonance stabilized. That is, they don't "work" like a normal double bond, but because they are alternated, that is, one yes and one no, they provide the molecule with its very special characteristics. Each carbon shows sp2 hybridization in benzene. These hybrids will be used both to form the bonds between carbons and the bonds between carbons and hydrogens. Each carbon also has an additional Pz orbital perpendicular to the molecular plane and with an electron housed inside it, which will be used to form π bonds.
Molecular reactivity
The typical reaction of benzene is that of aromatic substitution that follows two alternative paths:
- Electrophilic (by an electrophile attack)
- Free radicals (by attack of a free radical or free atom)
The most common aromatic substitution reactions are those caused by electrophilic reagents. The ability of benzene to act as an electron donor is due to the polarization of the benzene nucleus. The typical reactions of benzene are those of substitution. The most frequently used substitution agents are:
- Clone.
- Bromo.
- Nitric acid.
- Concentrated and hot sulfuric acid.
Halogenation
Chlorine and bromine give derivatives by substitution of one or more hydrogens from benzene, which are called aryl halides.
}}underbrace {{ce {C6H5Cl}}} _{mathrm {clorobenceno} }+{ce {HCl}}}" xmlns="http://www.w3.org/1998/Math/MathML">C6H6+Cl2Δ Δ C6H5Cl clorrorbencenor+HCl{displaystyle {ce {C6H6 + Cl2}}underbrace {{{ce {C6H5Cl}}}}} _{mathrm {chlorobenzene} }+{ce {HCl}}}}}}}underbrace {{ce {C6H5Cl}}} _{mathrm {clorobenceno} }+{ce {HCl}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/1f4876963bbf5a1d74bd4dcbd204f53c2f136c2a" style="vertical-align: -4.505ex; width:32.338ex; height:6.343ex;"/>
}}underbrace {{ce {C6H5Br}}} _{mathrm {bromobenceno} }+{ce {HBr}}}" xmlns="http://www.w3.org/1998/Math/MathML">C6H6+Br2Δ Δ C6H5Br brormorbencenor+HBr{displaystyle {ce {C6H6 + Br2}}underbrace {{ce {C6H5Br}}}} _{mathrm {bromobenceno} }+{ce {HBr}}}}}}}}underbrace {{ce {C6H5Br}}} _{mathrm {bromobenceno} }+{ce {HBr}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/0942201052b9eb2c7f374e13b1958af6e78cc99b" style="vertical-align: -4.505ex; width:33.898ex; height:6.343ex;"/>
Halogenation is favored by low temperatures and some catalyst, such as iron, aluminum trichloride or another Lewis acid, which polarizes the halogen for the reaction to take place. In the case of bromobenzene, FeBr3 is used as a catalyst.
Sulphonation
When benzene hydrocarbons are treated with concentrated sulfuric acid, which is a mixture of (H2SO4) and (SO3), characteristic compounds are formed that receive the name sulfonic acids. The reacting electrophile can be HSO3+ or SO3. It is the only reversible reaction we are considering.
- }}underbrace {{ce {C6H5SO3H}}} _{mathrm {{acute {a}}cido bencenosulf{acute {o}}nico} }+{ce {H2O}}}" xmlns="http://www.w3.org/1998/Math/MathML">C6H6+H2SO4H(SO3)Δ Δ C6H5SO3H a♪ ♪ cidorbencenorsulfor♪ ♪ nicor+H2O{displaystyle {ce {C6H6 + H2SO4H(SO3)}}{underbrace {{ce {C6H5SO3H}}}} _{mathrm {{acute {a}}cido bencenosulf{acute {o}nico}} }+{ce {H2O}}}{
}}underbrace {{ce {C6H5SO3H}}} _{mathrm {{acute {a}}cido bencenosulf{acute {o}}nico} }+{ce {H2O}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/e0a90d34664a6cf82dea3fad7c95779b2d74d876" style="vertical-align: -4.838ex; width:51.382ex; height:6.843ex;"/>
Nitration
Fuming nitric acid or a mixture of nitric and sulfuric acids, called a sulfonitric mixture, (one part nitric acid and three parts sulfuric), produces nitrated derivatives, by substitution. Sulfuric acid protonates nitric acid which is transformed into the positive nitronium ion (NO2+) which is the effective nitrating agent:
- }}underbrace {{ce {C6H5NO2}}} _{mathrm {nitrobenceno} }+{ce {H2O}}}" xmlns="http://www.w3.org/1998/Math/MathML">C6H6+MAN2(H2SO4)Δ Δ C6H5NO2 nitrorbencenor+H2O{displaystyle {ce {C6H6 + HONO2(H2SO4)}}{underbrace {{ce {C6H5NO2}}}}} _{mathrm {nitrobenceno} } }+{ce {H2O}}}}}}}
}}underbrace {{ce {C6H5NO2}}} _{mathrm {nitrobenceno} }+{ce {H2O}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/d10854e014e69581b34632751634e62fe83e1b52" style="vertical-align: -4.505ex; width:47.32ex; height:6.509ex;"/>
This process is carried out by reacting benzene with nitric acid and using sulfuric acid as a catalyst, a mixture known as sulfonitric, generating the nitronium ion NO2+, which acts as an electrophilic agent at a temperature between 50 to 60 °C, producing in this process nitro benzene and water
Combustion
Benzene is flammable and burns with a sooty flame, a characteristic property of most aromatic compounds and due to its high carbon content.
6 CO2 + 3 H2O}}}" xmlns="http://www.w3.org/1998/Math/MathML">C6H6+152O2Δ Δ 6CO2+3H2O{displaystyle {ce {C6H6 + 15/2 O2 - 2005 6 CO2 + 3 H2O}}}} 6 CO2 + 3 H2O}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/1e14edebc92081b99647ec53f3284b62c1358492" style="vertical-align: -1.171ex; width:33.894ex; height:3.509ex;"/>
Hydrogenation
The benzene ring can be reduced to cyclohexane, with catalytic hydrogenation (for example, using Raney nickel) at high pressure, thus maintaining the closed chain structure. The partial reduction can be carried out by means of the Birch method to form cyclohexadienes.
Friedel and Crafts synthesis (alkylation)
Benzene reacts with alkyl halides, in the presence of anhydrous aluminum chloride (AlCl3) as a catalyst, forming homologues.
}}underbrace {{ce {C6H5CH3}}} _{mathrm {tolueno} }+{ce {HCl}}}" xmlns="http://www.w3.org/1998/Math/MathML">C6H6+CH3ClΔ Δ C6H5CH3 torluenor+HCl{displaystyle {ce {C6H6 + CH3Cl)}underbrace {{{ce {C6H5CH3}}}}{mathrm {tolueno} }+{ce {HCl}}}}}}}}underbrace {{ce {C6H5CH3}}} _{mathrm {tolueno} }+{ce {HCl}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/d5359e4a8fd2a733da48289172d3b8ce927241c8" style="vertical-align: -4.505ex; width:36.535ex; height:6.343ex;"/>
The attack on the benzene ring by the +CH3 ion is similar to that carried out by the Cl+ ion in chlorination.
Wurtz–Fittig synthesis
It is a modification of the Wortz fat series. Homologs of benzene can be prepared by heating an ethereal solution of an alkyl and an aryl halide with sodium. This method has the advantage over the Friedel–Crafts method in that the structure of the product is known and normal long chains can be easily introduced.
- Benzene derivatives. Guiding influence of the elements that replace the benzene.
When a second substituent y is introduced in a benzene derivative of the type C6H5X, the position occupied by Y depends on the electronic character of the group X, which is already present in the kernel. The reaction products can be ortho and para or meta disubstituted and this depends on the rate of the substitution reaction at each of the three positions.
There are some orientation rules:
- Class I groups (electrons or deliverers) orient replacement to orthose positions. In this class one of the following groups can be found, OH, NH2, Cl, Br, I, F, CH2CI, SH, C6H5etc.
- Class II groups (electronic acceptors) orient replacement to target position. In this class they may include: N02, SO3H, CN, COOH, CHO, etc.
There is a simple orientation method for disubstituted derivatives that was established by Körner. It is often called the Körner 2,3,1 method. It is based on the principle that the introduction of a third substituent in a para compound provides a trisubstituted product, in the ortho isomer two and in the meta i> three. Körner applied this principle to establish the orientation of isomeric dibromobenzenes. He nitrated each of them and examined the number of nitrated products. The isomer that gave a single dibromo-nitrobenzene is the para; the one that gave two nitrated derivatives, the ortho, and the third one that gave three, is the meta compound.
Alkylbenzenes
Hydrocarbons such as toluene, ethylbenzene, phenol, etc., have an aliphatic and aromatic character. Benzene is nonpolar, as is methane, the dipole moment of each of these compounds being zero. However, toluene has a small dipole moment (approximately 0.4 D) with the negative charge on the nucleus and the positive charge on the methyl group. Alkylbenzenes undergo chlorination and bromination, either in the nucleus or in the side chain, depending on the reaction conditions. To name the relative positions of benzene, see Substitution Patterns in Aromatic Hydrocarbons.
Toxicity
Breathing very high levels of benzene can cause death, while low levels can cause drowsiness, dizziness, and rapid heartbeat or tachycardia. Eating or drinking high levels of benzene can cause vomiting, stomach irritation, dizziness, drowsiness or seizures, and ultimately death.
Long-term exposure to benzene shows up in the blood. Benzene has deleterious effects on the bone marrow and can cause a decrease in the number of red blood cells, leading to anemia. Benzene can also cause bleeding and damage the immune system, thus increasing the chances of contracting immunosuppressed infections.
The harmful effects of benzene increase with the consumption of alcoholic beverages.
Some studies on a sample of women who breathed high levels of benzene for several months have revealed that they had irregular menses, as well as a decrease in the size of their ovaries. It is not known whether benzene exposure affects the fetus during pregnancy. Several animal studies have described low birth weight and bone formation problems.
The US Department of Health and Human Services (DHHS) has determined that benzene is a known carcinogen to humans and other lactating mammals. Long-term exposure to high levels of benzene in the air can cause leukemia, as well as colon cancer.
In the body, benzene is transformed into products called metabolites. Certain metabolites can be measured in urine or feces. However, this test must be done promptly after exposure, and the test result does not indicate what concentration of benzene you were exposed to, as metabolites in urine may originate from other sources.
Uses of Benzene
Benzene is used as a constituent of motor fuels, solvents for greases, oils, and paints; in the photographic engraving of impressions; as a chemical intermediate in the manufacture of detergents, explosives, pharmaceuticals and paints; in the synthesis of other chemicals, such as styrene, cumene (in various resins), and cyclohexane (in nylon and synthetic fibers), in the manufacture of certain types of rubber, lubricants, and pesticides.
Contenido relacionado
Peroxide
Mass concentration (chemical)
Noble gases
