B lymphocyte
B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system through the secretion of antibodies. In addition, antigen-presenting B cells are also classified as antigen-presenting cells (APCs) and secrete cytokines. In mammals, B cells mature in the bone marrow, which is found in the nucleus of most bones. In birds, B cells mature in the bursa of Fabricius, a lymphoid organ where they were first discovered. by Chang and Glick, (B for bag) and not from bone marrow as is commonly believed. B cells, unlike the other two classes of lymphocytes, T cells and natural killer cells, express B cell receptors (BCRs) on their cell membrane. BCRs allow the B cell to bind to a specific antigen., against which it will initiate an antibody response.
Development
B cells develop from hematopoietic stem cells (HSCs) that originate in the bone marrow.
Hematopoietic stem cells (HSCs) differentiate first into multipotent progenitor (MPP) cells, then into common lymphoid progenitor (CLP) cells. i>, from English common lymphoid progenitor). Thereafter, its development into B cells occurs in several stages (shown in the image to the right), each marked by various patterns of gene expression and arrangements of immunoglobulin H chain and L chain gene loci, the latter because B cells undergo V(D)J recombination as they develop.
B cells undergo two types of selection while developing in the bone marrow, to ensure proper development. Positive selection occurs through antigen-independent signaling involving both the pre-BCR and the B cell receptor (BCR). If these receptors do not bind their ligand, B cells do not receive the proper signals and stop developing. Negative selection occurs through binding of the autoantigen to the B cell receptor (BCR); if the BCR can strongly bind to the autoantigen, then the B cell experiences one of four fates: clonal deletion, receptor editing, anergy, or ignorance (the B cell ignores the signal and continues development). This negative selection process leads to a state of central tolerance, in which mature B cells do not bind self antigens present in the bone marrow.
To complete development, immature B cells migrate from the bone marrow to the spleen as transitional B cells, passing through two transitional stages: T1 and T2. During their migration to the spleen and after entry of the spleen, they are considered T1 B cells. Within the spleen, T1 B cells pass into T2 B cells. T2 B cells differentiate into follicular (FO) B cells or marginal zone (MZ) B cells depending on signals received through the BCR and other receptors. Once differentiated, they are now considered mature B cells or B cells. naive.
Activation
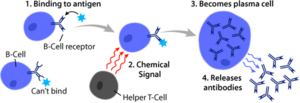
Activation of B cells occurs in secondary lymphoid organs (SLOs), such as the spleen and lymph nodes. After B cells mature in the bone marrow, they migrate through the blood to SLOs, which they receive a constant supply of antigen via circulating lymph. In SLO, B cell activation begins when the B cell binds to an antigen through its BCR. Although events that take place immediately after activation have not yet been fully determined, it is believed that B cells are activated according to the model of segregation kinetics, initially determined in T lymphocytes. This model denotes that prior to antigen stimulation, receptors diffuse through membrane contacting Lck and CD45 at equal frequency, resulting in a net balance of phosphorylation and nonphosphorylation. It is only when the cell comes into contact with an antigen-presenting cell that the larger CD45 is displaced due to the short distance between the two membranes. This allows net phosphorylation of the BCR and initiation of the signal transduction pathway. Of the three B cell subsets, FO B cells preferentially undergo T cell-dependent activation, whereas MZ B cells and B1 B cells preferentially undergo T cell-independent activation.
B cell activation is enhanced through the activity of CD21, a surface receptor in complex with the surface proteins CD19 and CD81 (the three are collectively known as the B cell coreceptor complex). a BCR binds to an antigen labeled with a C3 complement protein fragment, CD21 binds to the C3 fragment, ligates along with the bound BCR, and signals are transduced via CD19 and CD81 to lower the activation threshold of the cell.
T-cell dependent activation
Antigens that activate B cells with the help of T cells are known as T cell-dependent (TD) antigens and include foreign proteins. They are named as such because they cannot induce a humoral response in organisms that lack them. T cells. B cell responses to these antigens take several days, although the antibodies generated have a higher affinity and are more functionally versatile than those generated by independent T cell activation.
Once a BCR binds to a TD antigen, the antigen is taken up into the B cell via receptor-mediated endocytosis, degraded, and presented to T cells as peptide pieces in complex with TD molecules. MHC-II on the cell membrane. T helper (TH) cells, typically follicular helper T cells (TFH) recognize and bind these MHC-II peptide complexes through their T cell receptor (TCR). After binding of the TCR-MHC-II peptide, T cells express the CD40L surface protein, as well as cytokines such as IL-4 and IL-21. CD40L serves as a necessary costimulatory factor for B-cell activation by binding to the B-cell surface receptor CD40, which promotes B-cell proliferation, immunoglobulin class switching, and somatic hypermutation, in addition to maintain T-cell growth and differentiation. T-cell-derived cytokines bound by B-cell cytokine receptors also promote B-cell proliferation, immunoglobulin class switching, and somatic hypermutation, as well as guide T cell growth and differentiation. differentiation. After B cells receive these signals, they are considered activated.
Once activated, B cells participate in a two-step differentiation process that produces short-lived plasmablasts for immediate protection and long-lived plasma cells and memory B cells for persistent protection. The first step, known like the extrafollicular response, it occurs outside the lymphoid follicles but still in the SLO. During this step, activated B cells proliferate, can undergo an immunoglobulin class switch, and differentiate into plasmablasts that produce weak early antibodies, mostly of the IgM class. The second step involves the activated B cells entering a follicle. lymphoid cells and form a germinal center (GC), which is a specialized microenvironment where B cells undergo extensive proliferation, immunoglobulin class switching, and affinity maturation directed by somatic hypermutation. These processes are facilitated by T cells. FH within the GC and generate both high-affinity memory B cells and long-lived plasma cells. The resulting plasma cells secrete large amounts of antibodies and remain within the SLO or, more preferably, migrate to the bone marrow.
Independent activation of T cells
Antigens that activate B cells without the help of T cells are known as T cell-independent (TI) antigens and include foreign polysaccharides and unmethylated CpG DNA. They are named as such because they can induce a response humoral in organisms lacking T cells. The B cell response to these antigens is rapid, although the antibodies generated tend to have lower affinity and are less functionally versatile than those generated by T cell-dependent activation.
As with TD antigens, B cells activated by TI antigens require additional signals to complete activation, but instead of receiving them from T cells, they are provided through recognition and binding of a common microbial constituent to Toll-like receptors (TLRs) or extensive cross-linking of BCRs to repeated epitopes on a bacterial cell. B cells activated by TI antigens proliferate outside lymphoid follicles but still in SLO (GCs are not formed), possibly undergoing a change of the immunoglobulin class and differentiate into short-lived plasmablasts that produce early and weak antibodies, mainly of the IgM class, but also some populations of long-lived plasma cells.
B cell activation memory
Memory B cell activation begins with the detection and binding of its target antigen, which is shared by its parent B cell. Some memory B cells can activate without the help of T cells, such as certain virus-specific memory B cells, but others require help from T cells. Upon binding to antigen, the memory B cell takes up the antigen via receptor-mediated endocytosis, degrades it, and presents it to T cells as peptide pieces in complex with MHC-II molecules in the cell membrane. Memory helper T cells (TH), typically memory follicular helper T cells (TFH), which are derived from T cells activated with the same antigen recognize and bind to these MHC-II peptide complexes via their TCR. After TCR-MHC-II peptide binding and transmission of other signals from the memory TFH cell, the memory B cell becomes activated and differentiates into plasmablasts and plasma cells via an extrafollicular response or enters a germinal center reaction where they generate plasma cells and more memory B cells. It is unclear whether memory B cells undergo increased affinity maturation within these secondary GCs.
Types of B cells
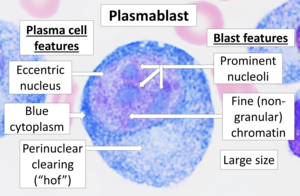
Plasmablast: A short-lived proliferating antibody-secreting cell that arises from B-cell differentiation. Plasmablasts are generated early in an infection and their antibodies tend to have a weaker affinity for their target antigen compared to plasma cells. Plasmablasts may be the result of T cell-independent activation of B cells or the extrafollicular response of T cell-dependent activation of B cells.
- Plasma cell: a secret cell of non-proliferative and long-life antibodies that arises from the differentiation of B cells. There is evidence that B cells first differentiate in a cell similar to plasmablasts, then differentiate in a plasma cell. Plasmatic cells are later generated in an infection and, compared to plasmablasts, have antibodies with greater affinity to their target antigen due to the ripening of affinity in the germinal center (GC) and produce more antibodies. Plasmatic cells usually result from the germinal center's reaction of T-cell-dependent B-cell activation, however, they may also be the result of activation of B-cells independent of T-cells.
- Lymphoplasmocytoid cell: a cell with a mixture of morphological characteristics of B lymphocytes and plasma cells that is believed to be closely related or is a subtype of plasma cells. This type of cells is found in premaligan and malignant plasma cell discrasias that are associated with the secretion of IgM monoclonal proteins; these discrasias include IgM monoclonal gammapathy of undetermined importance and Waldenström macroglobulinemia.
- Cell B Memory: Inactive B cell that arises from the differentiation of B cells. Its function is to circulate through the body and initiate a stronger and faster antibodies response (known as an amnestic secondary antibodies) if they detect the antigen that had activated their stem cells (the B cells of memory and their shared BCR cells share the same BCR, so they detect the same antigen). Memory B cells can be generated from T-cell-dependent activation through extrafolicular response and germ center reaction, as well as from independent activation of T-cells of B1 cells.
- Cell B-2:
- Follicular B Cell (FO) (also known as B-2 cell): the most common type of B cell and, when not circulating in the blood, is mainly found in the lymphoid follicles of the secondary lymphoid organs (SLO). They are responsible for generating most high affinity antibodies during an infection.
- Section B of the marginal zone (MZ): is mainly in the marginal area of the spleen and serves as the first line of defense against the pathogens transmitted by the blood, as the marginal area receives large amounts of blood from the general circulation. They may undergo both T-cell-dependent and T-cell-dependent activation, but preferably experience an independent T-cell activation.
- Cell B-1: stems from a different path of development of FO B cells and MZ B cells. In mice, predominantly populate peritoneal cavity and pleural cavity, they generate natural antibodies (uninfection-produced antibodies), defend themselves against mucous pathogens and mainly exhibit an independent activation of T cells. A real mouse B-1 cell counterpart has not been discovered in humans, although several cell populations similar to B-1 cells have been described.
- Regulation B (Breg): a type of B immunosuppressive cell that stops the expansion of pathogen pro-inflammatory lymphocytes through the secretion of IL-10, IL-35 and TGF-β. In addition, it promotes the generation of T-regulatory cells (Treg) by interacting directly with T-cells to bias their differentiation to Tregs. A common Breg cell identity has not been described and many Breg cell subsets have been found that share regulatory functions both in mice and in humans. It is currently unknown whether Breg cell subsets are linked to development and how exactly the differentiation occurs in a Breg cell. There is evidence that almost all types of B cells can be differentiated in a Breg cell through mechanisms involving inflammatory signals and BCR recognition.
B cell-related pathology
Autoimmune disease may result from abnormal recognition of autoantigens by B cells, followed by the production of autoantibodies. Autoimmune diseases where disease activity correlates with B cell activity include scleroderma, multiple sclerosis, systemic lupus erythematosus, type 1 diabetes, postinfectious IBS, and rheumatoid arthritis.
Malignant transformation of B cells and their precursors can cause a large number of cancers, including chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), hairy cell leukemia, follicular lymphoma, non-Hodgkin's lymphoma, lymphoma Hodgkin's and malignant plasma cell tumors such as multiple myeloma, Waldenström's macroglobulinemia, and certain forms of amyloidosis.
Epigenetics
A study investigating the methylome of B cells throughout their differentiation cycle, using whole genome bisulfite sequencing (WGBS), showed that there is hypomethylation from the earliest to the most differentiated stages. The largest methylation difference is between the stages of germinal center B cells and memory B cells. Furthermore, this study showed that there is a similarity between B cell tumors and long-lived B cells in their DNA methylation signatures.
Contenido relacionado
Bacteriology
Nosology
Tulip