Aromatic compound
The aromatic compounds are those chemical compounds (most commonly organic) that contain one or more rings with delocalized pi electrons around them. Unlike compounds that exhibit aromaticity, aliphatic compounds lack this delocalization. The term "aromatic" was assigned before the physical mechanism that determines aromaticity was discovered, and referred simply to the fact that many of these compounds have a sweet or pleasant odor; however, not all aromatic compounds are sweet-smelling and not all sweet-smelling compounds are aromatic. Aromatic hydrocarbons, or arenes, are aromatic organic compounds containing only carbon and hydrogen atoms. The configuration of six carbon atoms in aromatic compounds is called a "benzene ring", after the simple aromatic compound benzene, or a phenyl group when it is part of a larger compound.
Not all aromatic compounds are based on benzene; aromaticity can also manifest in Heterorenosthat follow the rule of Hückel (for monocyclic rings: when the number of their electrons π π {displaystyle pi } equals 4n+2{displaystyle 4n+2}Where n=0,1,2,3,...{displaystyle n=0,1,2,3,... !). In these compounds, at least one carbon atom is replaced by one of the heteroatoms: oxygen, nitrogen or sulfur. Examples of compounds other than benzene with aromatic properties are furan, a heterocyclic compound with a five-member ring that includes a single oxygen atom, and piridine, a heterocyclic compound with a six-member ring containing a nitrogen atom.
Benzene ring model
The benzene, C6H6{displaystyle {ce {C6H6}}}, is the less complex aromatic hydrocarbon and was the first to be named as such. The nature of its bond was first recognized by August Kekulé in the centuryXIX. Each carbon atom in the hexagonal cycle has four electrons to share. One goes to the hydrogen atom and one goes to each of the two neighboring carbons. This leaves an electron to share with one of the two neighbouring carbon atoms, thus creating a double link with one carbon and leaving a simple link with the other, which is why some representations of the benzene molecule describe it as a hexagon with simple alternation and double links.
Other representations of the structure show the hexagon with a circle inside it, to indicate that the six electrons are floating in delocalized molecular orbitals the size of the ring itself. This represents the equivalent nature of the six carbon-carbon bonds, all of bond order 1.5; the equivalence is explained by resonance forms. Electrons are visualized floating above and below the ring, and the electromagnetic fields they generate act to keep the ring flat.
General properties of aromatic hydrocarbons:
- Show aromaticity
- Carbon-hydrogen ratio is high
- Arden with a strong hollin yellow flame due to the high carbon-hydrogen ratio
- They are subjected to electrophilic replacement reactions and nucleophilic aromatic substitutions
The circular symbol of aromaticity was introduced by Sir Robert Robinson and his pupil James Armit in 1925 and popularized since 1959 by the Morrison & Boyd textbook on organic chemistry. The proper use of the symbol is discussed: some publications use it to any cyclical system π π {displaystyle pi }while others use it only for those systems π π {displaystyle pi } that obey Hückel's rule. Jensen argues that according to Robinson's original proposal, the use of the circle symbol should be limited to monocyclic systems of 6 electrons π π {displaystyle pi }. In this way, the circle symbol for a six-center link can be compared to the Y symbol of a three-center link of two electrons.
Synthesis of aromatics
A reaction that forms an aromatic compound from an unsaturated or partially unsaturated cyclic precursor is simply called aromatization. There are many laboratory methods for the organic synthesis of sandstones from non- sandal precursors. Many methods are based on cycladdic reactions. The trimerization of alkyns describes the cycle [chuckles]2+2+2]{displaystyle [2+2+2]} of three alkynos, in the reaction of Dötz a alkyno, carbon monoxide and a chrome carbene complex are the reagents. Diels-Alder reactions of alkynos with pyron or cyclopentadienone with carbon dioxide expulsion or carbon monoxide also form sandstone compounds. In Bergman's cycle, the reagents are an eniner plus a hydrogen donor.
Another set of methods is the aromatization of cyclohexanes and other aliphatic rings: the reagents are catalysts used in hydrogenation such as platinum, palladium, and nickel (reverse hydrogenation), quinones, and the elements sulfur and selenium.
Reactions
Aromatic ring systems are involved in many organic reactions.
Aromatic Substitution
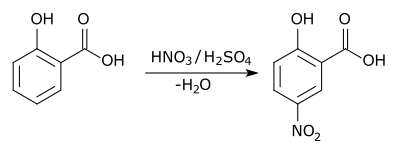
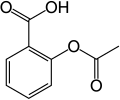
In aromatic substitution, one substituent on the arene ring, usually hydrogen, is replaced by another substituent. The two main types are electrophilic aromatic substitution when the active reagent is an electrophile and nucleophilic aromatic substitution when the reagent is a nucleophile. In radical nucleophilic aromatic substitution, the active reagent is a radical. An example of electrophilic aromatic substitution is the nitration of salicylic acid:
Mating Reactions
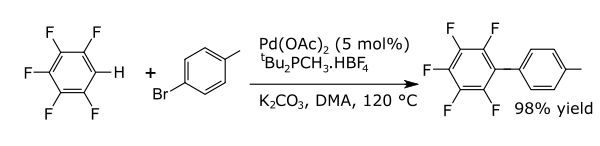
In coupling reactions, a metal catalyzes a coupling between two formal radical fragments. Common coupling reactions with arenes result in the formation of new carbon-carbon bonds, for example, alkylarenes, vinyl-arenes, biryls, new carbon-nitrogen bonds (anilines), or new carbon-oxygen bonds (aryloxy compounds). An example is the direct arylation of perfluorobenzenes.
Hydrogenation
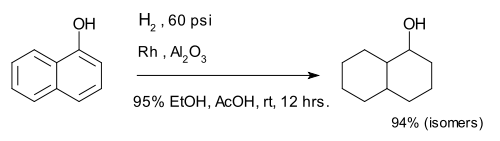
Hydrogenation of arenes creates saturated rings. The 1-naphthol compound is completely reduced to a mixture of decalin-ol isomers.
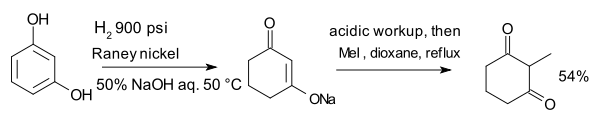
The compound resorcinol, hydrogenated with Raney nickel in the presence of aqueous sodium hydroxide forms an enolate that is alkylated with methyl iodide to 2-methyl-1,3-cyclohexanedione:
Cycloadditions
The cycloaddition reaction is not common. An unusual Diels-Alder thermal reactivity of arenes can be found in the Wagner-Jauregg reaction. Other photochemical cycloaddition reactions with alkenes occur via excimers.
Keromatization
In dearomatization reactions, the aromaticity of the reagent is permanently lost.
Benzene and derivatives of benzene
Benzene derivatives have one to six substituents attached to the central benzene nucleus. Examples of benzene compounds with only one substituent are phenol, which bears a hydroxyl group, and toluene with a methyl group. When there is more than one substituent present on the ring, their spatial relationship becomes important for which arene ortho, meta and para. For example, three isomers exist for cresol because the methyl group and hydroxyl group can be placed next to each other (ortho), one position apart from each other (meta) or two positions separated from each other (for). Xylenol has two methyl groups in addition to the hydroxyl group, and for this structure, there are 6 isomers.
- Representative aromatic compounds
The aromatic ring has the ability to stabilize loads. This is seen, for example, in the phenol (C6H5OH{displaystyle {ce {C6H5OH}}}}), which is acid in hydroxyl (OH{displaystyle {ce {OH}}}) as a load of this oxygen (aloxide) − − O− − {displaystyle {ce {-O-}}}) is partially delocalized in the benzene ring.
Other monocyclic aromatic hydrocarbon
Other monocyclic aromatic hydrocarbons include Cyclotetradecaheptaene or Cyclooctadecanoneene.
Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are aromatic hydrocarbons that consist of fused aromatic rings and do not contain heteroatoms or bear substituents. Naphthalene is the simplest example of PAH. PAHs are found in oil, coal, and tar deposits, and are produced as by-products of burning fuels (either fossil fuels or biomass). As contaminants, they are of concern because some compounds have been identified as carcinogenic, mutagenic, and teratogenic. PAHs are also found in cooked foods. Studies have shown that high levels of PAHs are found, for example, in meat cooked at high temperatures, such as broiling or grilling, and in smoked fish.
They are also found in the interstellar medium, in comets, and in meteorites, and are a candidate molecule to act as a basis for the oldest forms of life. In graphene, the HAP motif extends to large 2D sheets.
Contenido relacionado
Carbon(IV) oxide
Ionic radius
Pharmacy





![{displaystyle [2+2+2]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b5043d91151eb137d217c174831e274cfc29e495)