Antioxidant
An antioxidant is a molecule capable of retarding or preventing the oxidation of other molecules. Oxidation is a chemical reaction of electron transfer from a substance to an oxidizing agent. Oxidation reactions can produce radicals that start chain reactions that damage cells. Antioxidants terminate these reactions by removing radical intermediates and inhibit other oxidation reactions by oxidizing themselves. Because of this, antioxidants are often reducing agents such as thiols or polyphenols. Antioxidants are found in olives, garlic, brown rice, coffee, cauliflower, broccoli, eggplant, ginger, parsley, onion, citrus fruits, semolina, tomatoes, vine seed oil, tea, rosemary, among many other foods. The antioxidant capacity of some fruits, such as eggplants, is greater during their initial stages. They are also an important constituent of breast milk.
Although oxidation reactions are crucial to life, they can also be detrimental; therefore plants and animals maintain complex systems of multiple types of antioxidants, such as glutathione, vitamin C, and vitamin E, as well as enzymes such as catalase, superoxide dismutase, and various peroxidases. Low levels of antioxidants or inhibition of antioxidant enzymes cause oxidative stress and can damage or kill cells.
Oxidative stress has been associated with the pathogenesis of many human diseases. For this reason, pharmacology intensively studies the use of antioxidants, particularly as a treatment for strokes and neurodegenerative diseases. However, it is unknown whether oxidative stress is the cause or the consequence of such diseases. Antioxidants are also widely used as ingredients in dietary supplements in the hope of maintaining health and preventing diseases such as cancer and ischemic heart disease. Although some studies have suggested that antioxidant supplements have health benefits, other large clinical trials have found no benefit to the formulations tested, and over-supplementation may be harmful. In addition to these medical applications, antioxidants have many industrial applications, such as food and cosmetic preservatives and the prevention of rubber and gasoline degradation.
History
The term antioxidant was originally used to refer specifically to a chemical that prevented the consumption of oxygen. At the end of the 19th century and at the beginning of the XX, extensive studies were devoted to the applications of antioxidants in important industrial processes, such as the prevention of metal corrosion, the vulcanization of rubber, and the polymerization of fuels in the formation of slag in engines internal combustion.
Early research on the role of antioxidants in biology focused on their use in preventing the oxidation of unsaturated fats, which is the cause of rancidity. Antioxidant activity could be measured simply by placing the fat in a closed container with oxygen and measuring the rate of oxygen consumption. However, it was the identification of vitamins A, C, and E as antioxidants that revolutionized the field and led to the elucidation of the importance of antioxidants in the biochemistry of living organisms.
The possible mechanisms of action of antioxidants were first investigated when it was recognized that a substance with antioxidant activity is likely to be one that readily oxidizes itself. Research into how vitamin E prevents the process of Lipid peroxidation led to the identification of antioxidants as reducing agents that prevent oxidative reactions, often scavenging reactive oxygen species before they can damage cells.
The oxidative challenge in nature
A paradox in metabolism is that while the vast majority of complex life requires oxygen for its existence, oxygen is a highly reactive molecule that harms living things by producing reactive oxygen species. Therefore, organisms possess a complex network of antioxidant metabolites and enzymes that work together to prevent oxidative damage to cellular components such as DNA, proteins, and lipids. Antioxidant systems generally prevent these reactive species from being formed or remove them prior to that can damage vital cell components.
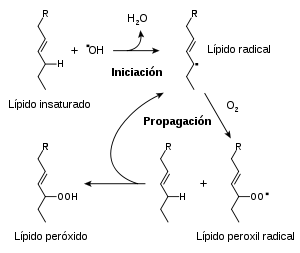
Reactive oxygen species produced in cells include hydrogen peroxide (H2O2), hypochlorous acid (HClO), and free radicals such as the hydroxyl radical (OH) and the superoxide radical (O2 −). The hydroxyl radical is particularly unstable and reacts rapidly and nonspecifically with most biological molecules. This species is produced from hydrogen peroxide in metal-catalyzed redox reactions such as the Fenton reaction. These oxidants can damage cells by starting chemical chain reactions such as lipid peroxidation or by oxidizing DNA or proteins. Damage to DNA they can cause mutations and possibly cancer if not reversed by DNA repair mechanisms, while protein damage causes enzyme inhibition, denaturation, and protein degradation.
The use of oxygen as part of the process to generate metabolic energy produces reactive oxygen species. In this process, superoxide anion is produced as a byproduct of various steps in the electron transport chain. Particularly important is the reduction of coenzyme Q in complex III, as a highly reactive free radical is formed as an intermediate (Q·−). This unstable intermediate can lead to a loss of electrons as they jump directly to molecular oxygen and form the superoxide anion instead of moving through the well-controlled series of reactions of the electron transport chain. In a similar system of reactions in plants reactive oxygen species are also produced during photosynthesis under high light intensity conditions. This effect is partly offset by the involvement of carotenoids in photoinhibition, which implies that these antioxidants react with the over-reduced forms of the centers photosynthetic reaction cells and thereby prevent the production of superoxide.
Another process that produces reactive oxygen species is lipid oxidation that occurs as a consequence of the production of eicosanoids. However, cells are provided with mechanisms that prevent unnecessary oxidation. The oxidative enzymes in these biosynthetic pathways are coordinated and highly regulated.
Metabolites
Description
Antioxidants are classified into two broad groups, depending on whether they are soluble in water (water soluble) or lipid (liposoluble). In general, water-soluble antioxidants react with oxidants in the cell cytoplasm and blood plasma, whereas fat-soluble antioxidants protect cell membranes against lipid peroxidation. These compounds can be synthesized in the body or obtained from the diet. Different antioxidants are present in a wide range of concentrations in body fluids and tissues, with some such as glutathione or ubiquinone present mostly within cells, while others such as uric acid are more evenly distributed throughout the body. of the body.
The relative importance and interactions between these different antioxidants constitutes a complex area, with various metabolites and enzyme systems having synergistic and interdependent effects on one another. The action of an antioxidant may depend on the appropriate function of other members of the antioxidant system. The amount of protection provided by any antioxidant depends on its concentration, its reactivity towards reactive oxygen species, and the state of the antioxidants with which it interacts.
Some compounds contribute to antioxidant defense by chelating transition metals and preventing them from catalyzing the production of free radicals in the cell. Particularly important is the ability to sequester iron, which is the function of iron-binding proteins such as transferrin and ferritin. Selenium and zinc are commonly mentioned as antioxidant nutrients but these chemicals do not have any antioxidant action themselves. themselves but are required for the activity of some antioxidant enzymes.
| Antioxidant metabolite | Solubility | Human serum concentration (μM) | Concentration in liver tissue (μmol/kg) |
|---|---|---|---|
| Ascorbic acid (vitamin C) | Water | 50 - 60 | 260 (man) |
| Glutathion | Water | 325 - 650 | 6.400 (man) |
| Lipoic acid | Water | 0.1 - 0.7 | 4-5 (rata) |
| Uric acid | Water | 200 - 400 | 1,600 (man) |
| Carotenos | Liar | β-carotene: 0.5 – 1
(vitamin A): 1 - 3 | 5 (man, total carotenoids) |
| α-tocopherol (vitamin E) | Liar | 10 - 40 | 50 (man) |
| Ubiquinol (coenzyme Q) | Liar | 5 | 200 (man) |
Ascorbic acid
Ascorbic acid or vitamin C is a monosaccharide antioxidant found in animals and plants. Since it cannot be synthesized by humans and must be obtained from the diet, it is a vitamin. Most other animals can produce this compound in their bodies and do not require it in their diets. In cells, it is maintained in their reduced form by reaction with glutathione, which can be catalyzed by protein disulfide isomerase and glutaredoxins. Ascorbic acid is a reducing agent and can reduce and thereby neutralize reactive oxygen species such as hydrogen peroxide. In addition to its direct antioxidant effects, ascorbic acid is also a substrate for the antioxidant enzyme ascorbate peroxidase, a function that is particularly important in stress resistance in plants.
Glutathione
Glutathione is a cysteine-containing peptide and is found in most forms of aerobic life. It is not required in the diet and is synthesized in cells from its constituent amino acids. Glutathione has antioxidant characteristics as it the thiol group on its cysteine portion is a reducing agent and can be reversibly oxidized and reduced. In cells, glutathione is maintained in a reduced form by the enzyme glutathione reductase and in turn reduces other metabolites and enzyme systems as well as reacts directly with oxidants. Due to its high concentration and central role in maintaining the redox state of the cell, glutathione is one of the most important cellular antioxidants.
Melatonin
Melatonin is a powerful antioxidant that can easily cross cell membranes and the blood-brain barrier. Unlike other antioxidants, melatonin does not undergo redox cycling, which is the ability of a molecule to undergo reduction and oxidation many times. Redox cycling allows other antioxidants (such as vitamin C) to act as pro-oxidants and promote the formation of free radicals. Melatonin, once oxidized, cannot be reduced to its previous state because it forms several stable end products once it reacts with free radicals. Therefore, it has been referred to as a terminal (or suicidal) antioxidant.
Tocopherols and tocotrienols
Vitamin E is the collective name for a system of eight related tocopherols and tocotrienols, which are fat-soluble antioxidant vitamins. Of these, α-tocopherol has been widely studied as it has the highest bioavailability and is preferentially absorbed by the body. and metabolizes this form. The α-tocopherol form is the most important of the fat-soluble antioxidants and protects cell membranes against oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction. This removes the intermediate forms of free radicals and prevents the propagation of the chain reaction from continuing. The oxidized radicals of α-tocopheroxyl produced in this process can be recycled back to the active reduced form through reduction by ascorbate, retinol, or ubiquinol. The functions of the other forms of vitamin E are less well understood., although γ-tocopherol is a nucleophile that can react with electrophilic mutagens and tocotrienols may have a specialized role in neuroprotection.
Carotenoids
Carotenoids are among the most common natural pigments and more than 1100 different compounds have been characterized so far. Carotenoids are responsible for many of the red, yellow, and orange colors of plant leaves, fruits, and flowers, as well as the color of some insects, birds, fish, and crustaceans. They can only be synthesized by plants, fungi, bacteria and algae, however many animals incorporate them through the diet. Two important dietary carotenoids are lycopene and β-carotene. These are involved in the scavenging of two of the reactive oxygen species, singlet oxygen and the peroxyl radical. They are also effective in deactivating electronically excited molecules which are involved in the generation of both radicals and singlet oxygen itself. The quenching of singlet oxygen by carotenoids occurs through both physical and chemical quenching. The interaction of carotenoids with singlet oxygen depends mainly on physical quenching, which implies direct energy transfer between both molecules. The energy from singlet oxygen is transferred to the carotenoid producing molecular triplet oxygen (in its ground state) and excited carotene. The carotenoid returns to its ground state, dissipating this energy through interaction with the surrounding solvent. In contrast to physical quenching, the chemical reactions between singlet oxygen and carotenoids are of minor importance, contributing less than 0.05% of the total quenching rate. Since the carotenoids remain intact during physical quenching, from singlet oxygen they can be reused several times in these quenching cycles. β-carotene and other carotenoids are the most efficient natural quenchers for singlet oxygen. Their activity as quenchers is related to the number of conjugated double bonds present in the molecule. Carotenoids efficiently scavenge peroxyl radicals, especially when oxygen tension is low. The deactivation of peroxyl radicals probably depends on radical adduct formation forming a resonance-stabilized radical central carbon Cantrell et al (2003), reported the ability of six dietary carotenoids (β-carotene, lycopene, zeaxanthin, astaxanthin, canthaxanthin and lutein) to quench singlet oxygen in a model of cell membranes, where singlet oxygen was generated in both the aqueous and lipid phases, finding that lycopene and β-carotene exhibited the fastest quenching rate, being the lutein the least efficient. The other carotenoids had intermediate constants. Bando et al (2004) carried out an experiment using mice fed with β-carotene to determine if it serves as an antioxidant in skin exposed to UV-A rays, acting as a singlet oxygen quencher, finding that β-carotene Dietary accumulates in the skin and acts as a protective agent against oxidative damage induced by UV-A radiation, through singlet oxygen quenching. Dietary β-carotenes accumulate in the skin and act as protective agents against oxidative damage induced by UV-A radiation, through singlet oxygen quenching.
Polyphenols
Polyphenols are low molecular weight phytochemicals that are essential for humans. These are one of the secondary metabolites of plants, the most numerous and distributed throughout the plant, with more than 800 known structures today. Natural polyphenols can range from simple molecules (phenolic acid, hydroxytyrosol, phenylpropanoids, flavonoids) to highly polymerized compounds (lignins, tannins). Flavonoids represent the most common and widely distributed subgroup and among them flavonols are the most widely distributed. Being widely distributed in the plant kingdom, they constitute an integral part of the diet. Polyphenols have an ideal chemical structure for activity as free radical scavengers. Its property as an antioxidant comes from its great reactivity as donors of electrons and hydrogens and from the ability of the radical formed to stabilize and delocalize the unpaired electron (terminates the chain reaction) and from its ability to chelate transition metal ions. Polyphenols have a hydrophilic portion and a hydrophobic portion, so they can act against ROS that are produced in both hydrophobic and aqueous media. Its antioxidant capacity is directly related to the degree of hydroxylation of the compound. Flavonoids have a powerful antioxidant action in vitro, being able to scavenge a wide range of reactive oxygen, nitrogen and chlorine species, such as superoxide, hydroxyl radical, the peroxyl radical, hypochlorous acid, acting as reducing agents. They can also chelate transition metal ions. Soobrattee et al (2005), evaluated the antioxidant capacity of different polyphenols, finding that compared with physiologically active antioxidants (glutathione, α-tocopherol, ergothioneine) and synthetic ones (trolox, BHT, BHA), these compounds exhibited greater efficacy as antioxidants. Roginsky (2003), measuring the antioxidant activity of several natural polyphenols during the oxidation of methyl linoleate, found that all the polyphenols studied showed a pronounced antioxidant activity, considering that the underlying molecular mechanism of the antioxidant activity of polyphenols is that of acting by breaking the chain reaction. Polyphenols with two adjacent hydroxyl groups or any other chelating structure can bind transition metals. Polyphenols act as consumers of the hydroxyl radical, peroxynitrite and hypochlorous acid, acting as reducing agents.
Pro-oxidant activities
Antioxidants that are reducing agents can also act as pro-oxidants. For example, vitamin C has antioxidant activity when it reduces oxidizing substances such as hydrogen peroxide, however it can also reduce metal ions leading to the generation of free radicals through the Fenton reaction.
- 2 Fe3+ + Ascorbato → 2 Fe2+ + Dehydroascorbato
- 2 Fe2+ + 2 H2O2 → 2 Fe3+ + 2 OH· + 2 OH−
The relative importance of antioxidant and pro-oxidant activities is an area of current research, but vitamin C, for example, appears to have a major antioxidant action in the body. However, there is less data available for other dietary antioxidants, such as the antioxidant polyphenols, zinc, and vitamin E.
Enzyme systems
Description
As with chemical antioxidants, cells are protected against oxidative stress by a network of antioxidant enzymes. Superoxide released by processes such as oxidative phosphorylation is first converted to hydrogen peroxide and immediately reduced to water. This detoxification pathway is the result of multiple enzymes with superoxide dismutase catalyzing the first step and then catalases and various peroxidases removing hydrogen peroxide. As with antioxidant metabolites, the contributions of these enzymes can be difficult to separate from one another, but the generation of transgenic mice lacking only one antioxidant enzyme can be informative.
Superoxide dismutase, catalase and peroxiredoxins
Superoxide dismutases (SODs) are a class of closely related enzymes that catalyze the passage of superoxide anion into oxygen and hydrogen peroxide. SODs are present in nearly all aerobic cells and in fluid extracellular. Superoxide dismutase enzymes contain metal ions as cofactors which, depending on the isoenzyme, can be copper, zinc, manganese, or iron. In humans, zinc/copper SODs are present in the cytosol, while manganese SODs are found in mitochondria. A third form of SODs also exists in extracellular fluids, containing both copper and zinc in their cells. active sites. The mitochondrial isoenzyme appears to be the most biologically important of these three, as mice lacking this enzyme die shortly after birth. In contrast, mice lacking zinc/copper SODs are viable although decreased their fertility, whereas mice without extracellular SODs have minimal defects. In plants, SOD isoenzymes are present in the cytosol and mitochondria, with iron SODs found in chloroplasts and absent in vertebrates and yeast.
Catalases are enzymes that catalyze the conversion of hydrogen peroxide to water and oxygen using iron or manganese as a cofactor. This protein is located in the peroxisomes of most eukaryotic cells. Catalase is an unusual enzyme since although hydrogen peroxide is its only substrate, it follows a ping-pong mechanism. Its cofactor is oxidized by a hydrogen peroxide molecule and then regenerated by transferring the bound oxygen to a second substrate molecule. Despite its obvious importance in the removal of hydrogen peroxide, humans with genetic catalase deficiency – "acatalasemia" – or mice genetically engineered to lack catalase completely suffer few negative effects.
Peroxiredoxins are peroxidases that catalyze the reduction of hydrogen peroxide, organic hydroperoxide, and peroxynitrite. They are divided into three classes: the typical 2-cysteine peroxiredoxins; the atypical 2-cysteine peroxiredoxins; and the 1-cysteine peroxiredoxins. These enzymes share the same basic catalytic mechanism, in which a redox-active cysteine at the active site is oxidized to a sulfenic acid by the peroxide substrate.
Peroxiredoxins appear to be important in antioxidant metabolism, as mice lacking peroxiredoxin 1 or 2 have shortened lifespans and suffer from hemolytic anemia, while plants use peroxiredoxins to remove hydrogen peroxide generated in chloroplasts.
Thioredoxin and glutathione systems
The thioredoxin system contains the 12-kDa protein thioredoxin and its companion thioredoxin reductase.
Thioredoxin-related proteins are present in all organisms sequenced, with plants such as Arabidopsis thaliana having a particularly large diversity of isoforms. The thioredoxin active site consists of two neighboring cysteines, as part of a highly conserved CXXC structural motif that can cycle between an active form of the reduced dithiol and the oxidized form of the disulfide. In its active state, thioredoxin acts as an efficient reducing agent, removing reactive oxygen species and maintaining other proteins in their reduced state. After being oxidized, active thioredoxin is regenerated by the action of thioredoxin reductase, using NADPH as the electron donor.
The glutathione system includes glutathione, glutathione reductase, glutathione peroxidase, and glutathione S-transferase. This system is found in animals, plants, and microorganisms. Glutathione peroxidase is an enzyme that contains four selenium cofactors that catalyze the breakdown of hydrogen peroxide and organic hydroperoxides. There are at least four different isoenzymes of glutathione peroxidase in animals. Glutathione peroxidase 1 is the most abundant and is a very efficient scavenger of hydrogen peroxide, while glutathione peroxidase 4 is the most active with lipid hydroperoxides. Surprisingly, glutathione peroxidase 1 is not essential, as mice lacking this enzyme have normal lifespans, but are hypersensitive to induced oxidative stress. In addition, glutathione S-transferases are another class of glutathione-dependent antioxidant enzymes. which show high activity with lipid peroxides. These enzymes are found at particularly high levels in the liver and also serve in detoxification metabolism.
Oxidative stress and diseases
Oxidative stress is thought to contribute to the development of a wide range of diseases including Alzheimer's disease, Parkinson's disease, pathologies caused by diabetes, rheumatoid arthritis, and neurodegeneration in diseases of motor neurons. In many of these cases, it is not clear whether the oxidants trigger the disease, or if they are produced as a consequence of the disease and cause disease symptoms; as a plausible alternative, a neurodegenerative disease may result from transport Defective axonal of mitochondria carrying out oxidation reactions. One case in which this fits is the particularly well understood role of oxidative stress in cardiovascular disease. Here, the oxidation of low-density lipoprotein (LDL) seems to drive the process of atherogenesis, which leads to atherosclerosis, and ultimately to cardiovascular disease. Similarly, numerous studies have observed that oxidative stress promotes the apoptosis by different signaling pathways.
A low-calorie diet prolongs average and maximum lifespans in many animals. This effect may imply a reduction in oxidative stress. While there is good evidence supporting a role of oxidative stress in aging in model organisms such as Drosophila melanogaster and Caenorhabditis elegans, the evidence in mammals is less clear. Diets rich in fruits and vegetables, which have high levels of antioxidants, promote health and reduce the effects of aging,[citation needed] However, antioxidant vitamin supplementation has no discernible effect on the aging process, so the effects of fruits and vegetables may not be related to their antioxidant content.
Health effects
Treatment of diseases
The brain is unique in that it is highly vulnerable to oxidative damage due to its high metabolic rate and elevated levels of polyunsaturated lipids that are the targets of lipid peroxidation. Antioxidants are therefore commonly used in medicine to treat various forms of brain injury. Superoxide dismutase analogues such as sodium thiopentate and propofol are used to treat reperfusion injury and traumatic brain injury, while the experimental drug NXY-059 and ebselen are used to treat stroke.. These compounds appear to prevent oxidative stress in neurons and prevent apoptosis and neurological damage. Antioxidants are also being investigated as potential treatments for neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis.
Disease prevention
Antioxidants can nullify the damaging effects of free radicals on cells, and people with a diet of fruits and vegetables rich in polyphenols and anthocyanins have a lower risk of cancer, heart disease, and some neurological diseases. This observation suggested that these compounds could prevent diseases such as macular degeneration, suppressed immunity due to poor nutrition, and neurodegeneration, which are caused by oxidative stress. However, despite the clear role of oxidative stress in In cardiovascular disease, controlled studies using antioxidant vitamins have not shown any clear reduction in the progression or risk of heart disease. This suggests that other substances in fruits and vegetables (possibly flavonoids) at least partially explain the better health cardiovascular disease of those who consume more fruits and vegetables.
The oxidation of low-density lipoproteins in the blood is thought to contribute to heart disease, and initial observational studies found that people taking vitamin E supplements had lower risks of developing heart disease. There have been at least seven large clinical trials conducted to test the effects of antioxidant supplementation with vitamin E, in doses ranging from 50 to 600 mg per day. However, in none of these trials was a statistically significant effect of vitamin E found on the total number of deaths or on deaths due to heart disease.
While several trials have investigated supplements with high doses of antioxidants, the study "Supplémentation en Vitamines et Mineraux Antioxydants" (SU.VI.MAX) tested the effect of supplementation with doses comparable to those of a healthy diet. More than 12,500 men and women in France took both low doses of antioxidates (120 mg ascorbic acid, 30 mg vitamin E, 6 mg beta-carotene, 100 μ μ {displaystyle mu }g of selenium, and 20 mg of zinc) or placebo pills for an average of 7.5 years. Researchers found that there was no significant statistical effect of antioxidants on average life expectancy, cancer, or heart disease. However, an analysis of a subgroup showed a reduction of 31% in the risk of cancer in men, but not in women.
The production of natural antioxidants and antioxidants obtained from food is not sufficient for most people, for this reason many food and nutraceutical companies sell antioxidant formulations as dietary supplements and these are widely consumed in industrialized countries. These supplements may include specific antioxidant chemicals, such as resveratrol (from grapeseed), antioxidant combinations, such as "ACES" products containing beta-carotene (provitamin A), vitamin C, vitamin E and Selenium, or herbs special ones known to contain antioxidants, such as green tea and jiaogulan. Although some levels of antioxidant vitamins and minerals in the diet are necessary for good health, there is considerable question as to whether antioxidant supplements are beneficial, and if so, which antioxidants and in what amounts.
Antioxidant therapy is one of the topics with the greatest research activity in recent years, in which the presence of various nanomaterials with potential pharmacological properties has begun to be added.
Physical exercise
During exercise, oxygen consumption can increase by a factor of greater than 10. This leads to a large increase in oxidant production and results in damage that contributes to muscle fatigue during and after exercise. The inflammatory response that occurs after strenuous exercise is also associated with oxidative stress, especially in the 24 hours after an exercise session. The immune system's response to damage caused by exercise peaks 2 to 7 days after exercise, the adaptation period during which the result of increased fitness is greatest. During this process free radicals are produced by neutrophils to remove damaged tissue. As a result, elevated levels of antioxidants have the potential to inhibit recovery and adaptation mechanisms.
Evidence of the benefits of antioxidant supplementation in vigorous exercise has yielded conflicting results. There is strong evidence that one of the adaptations resulting from exercise is the strengthening of the body's antioxidant defences, particularly the glutathione system, to cope with increased oxidative stress. It is possible that this effect may be to some extent a protection against diseases that are associated with oxidative stress, which could provide a partial explanation for the lower incidence of the most common diseases and an improvement in the health of people who exercise regularly.
However, no benefits have been observed in athletes taking vitamin A or E supplements. For example, despite their key role in preventing membrane lipid peroxidation, in 6 weeks of vitamin A supplementation E no effect on muscle damage is observed in marathon runners. Although there appears to be no increased vitamin C requirement in athletes, there is some evidence that vitamin C supplementation increases the amount of strenuous exercise that is performed. can do and that supplementing with vitamin C before these exercises may reduce the amount of muscle damage. However, other studies have found no such effects and some suggest that supplementation with amounts as high as 1,000 mg inhibits recovery.
Adverse effects
Relatively strong reducing acids can have negative effects on nutrition by binding with dietary minerals such as iron and zinc in the gastrointestinal tract, preventing them from being absorbed. Notable examples include oxalic acid, tannins and phytic acid, which are found in high amounts in vegetarian diets. Iron and calcium deficiencies are frequent in the diets of developing countries, where the diet is less meaty and there is a high consumption of phytic acid of beans and whole grain flatbread.
| Food | Reducer acid present |
|---|---|
| Chocolate, spinach, nabo and ruibarbo. | Oxalic acid |
| Whole grains, corn, legumes. | Phytic acid |
| Tea, beans, cabbage. | Taninos |
Nonpolar antioxidants such as eugenol, an important component of clove oil, have toxicity limits that can be exceeded by misuse of undiluted essential oils. Toxicity associated with high doses of water-soluble antioxidants such as ascorbic acid is much less common, as these compounds can be rapidly excreted in the urine. Very high doses of some antioxidants can have long-term deleterious effects. Analyzes of the beta-carotene and retinol efficacy trials (CARET) in patients with lung cancer have shown that smokers who take beta-carotene supplements increase their chances of developing this type of cancer. Subsequent studies have confirmed these negative effects in smokers caused by beta-carotene.
These deleterious effects may also be seen in non-smokers, according to a recent meta-analysis of data including data from approximately 230,000 patients showing that supplementation with beta-carotene, vitamin A, or vitamin E is associated with increased mortality, but no significant effect is seen with vitamin C.
No health risks were observed when all randomized studies were examined together, but an increase in mortality was detected only when trials of high quality and low systematic error were analyzed separately. However, as most of these trials dealt with people who were elderly, or already suffering from disease, these results may not be applicable to the general population. These results are consistent with some preceding meta-analyses, which also suggested that supplementation with vitamin E increased mortality, and that antioxidant supplements increase the risk of colon cancer. However, the results of this meta-analysis are inconsistent with other studies, such as the SU.VI.MAX trial, which suggest that antioxidants do not have no effect on causes of mortality. Overall, the large number of clinical trials conducted on antioxidant supplements suggest that either these products have no effect on health or cause a small increase in mortality in the elderly or in vulnerable population groups.
While antioxidant supplementation is widely used in attempts to prevent the development of cancer, it has been proposed that antioxidants may, paradoxically, interfere with cancer treatments. Cancer cells' environment causes high levels of oxidative stress, making these cells more susceptible to further treatment-induced oxidative stress. As a consequence, by reducing redox stress in cancer cells, antioxidant supplements are thought to decrease the efficacy of radiotherapy and chemotherapy. However, this concern does not appear to be valid, as it has been addressed by multiple clinical trials that indicate that antioxidants may be neutral or beneficial in the treatment of cancer.
Controversies
There are not a few experts who maintain critical positions on the supposed beneficial effects attributed to antioxidant supplements. No one disputes today that there is evidence that fruits and vegetables that contain antioxidants have certain beneficial effects on different aspects of health. Consequently, it is accepted as a recommendation for healthy consumption to regularly take these natural products, fruits and vegetables.
But affirming, by extension, that the consumption of antioxidant supplements has these same beneficial effects is scientifically refutable. Undoubtedly, more and better scientific evidence is needed on this issue:
- The American Heart Association recognizes that there is no evidence that antioxidant supplements have any role in cardiovascular risk prevention, and does not recommend them, and even mentions that some limited data indicate certain risks associated with these products.
- The National Cancer Institute, in the same way, emphasizes that evidence to claim that antioxidant supplements play some role in cancer prevention is insufficient, and that therefore a healthy diet based on plant foods should be followed without resorting to supplements.
Measurement and levels in food
The measurement of antioxidants is not a straightforward process, as this is a diverse group of compounds with various reactivities to various reactive oxygen species. In food technology, Oxygen Radical Absorbance Capacity (ORAC) has become the current industry standard for determining the antioxidant capacity of foods, juices, and food additives. Other tests Measurement methods include the Folin-Ciocalteu reagent and the trolox equivalent antioxidant capacity assay. In medicine, a range of different assays are used to determine the antioxidant capacity of blood plasma and of these, the ORAC assay is the most reliable.
Antioxidants are found in varying amounts in foods such as vegetables, fruits, grain cereals, legumes, and nuts. Some antioxidants such as lycopene and ascorbic acid can be destroyed if stored for a long time, or by prolonged cooking. Other antioxidant compounds are more stable, for example polyphenol antioxidants in foods such as cereals, whole wheat, and tea.. In general, processed foods contain fewer antioxidants than fresh and raw foods, since the preparation processes expose the food to oxygen. There is a growing market for functional foods, which has led to the emergence of fortified products. in antioxidants, such as various margarines or olive oil enriched with lycopene.
| Antioxidant compounds | Food |
|---|---|
| Vitamin C (ascorbic acid) | Fruits and vegetables |
| Vitamin E (tocopherols, tocotrienoles) | Vegetable oils |
| Polyphenoid antioxidants (resveratrol, flavonoids) | Tea, coffee, soy, fruit, chocolate, oregano and red wine. |
| Carotenoids (licone, carotene) | Fruits and vegetables |
Some antioxidants are produced in the body and are not absorbed in the intestine. An example is glutathione, which is produced from amino acids. While any glutathione in the intestines is cleaved to release cysteine, glycine, and glutamic acid before being absorbed, even large oral doses have little effect on glutathione concentration in the body. Ubiquinol (coenzyme Q) is also poorly absorbed. in the intestines and is produced in humans via the mevalonate route.
Uses in technology
Antioxidants in food
The oxidation of food, or rancidity, is the second cause of deterioration, after alteration by microorganisms. Consequently, antioxidants are used to preserve food. Exposure to oxygen and sunlight are two of the main factors that cause oxidation of food, so food is protected by keeping it in the dark and in oxygen-impermeable containers. In the preservation of unprocessed vegetables, as oxygen is also important for plant respiration, storing them in anaerobic conditions produces off-flavors and colors. Therefore, packaging of fresh fruits and vegetables contains an atmosphere of ~8 oxygen. %. Enzymes may also be involved in oxidation. Its action is prevented by scalding.
Oxidation mainly affects unsaturated fatty acids, so the more unsaturated the fats in a food, the easier it is for them to oxidize. Oxidation is produced by a chain reaction of free radicals, which is accelerated by the presence of metal ions with two stable valences, especially iron and copper. This reaction can also occur in frozen foods. Antioxidants naturally present or added to food can act in four ways:
- Eliminating oxygen by reacting with it, like ascorbic acid and Eritrean.
- Cutting the chain propagation reaction, stabilizing free radicals. Tocopherols (E306), propyl galato (PG, E310), ter-butilhydroquinone (TBHQ), butil hydroxianisol (BHA, E320) and hydroxytoluene butil (BHT, E321), as well as various antioxidants present in spices, such as carnosic acid of romer.
- Kidnapping metals that can act as catalysts. This is the mechanism by which citric acid and EDTA operate.
- Using single oxygen quenching, a mechanism for which carotenoids operate.
Industrial use
Some antioxidants are added to industrial products. A common use is as a stabilizer in fuels and lubricants to prevent oxidation, and in gasoline to prevent polymerization that leads to residue formation in engines. They are also used to prevent oxidative degradation of rubber, plastics, and glues which causes a loss of strength and flexibility of these materials. Antioxidant preservatives are also added to fat-based cosmetics such as lipsticks and moisturizers to prevent rancidity.
| Additive | Components | Applications |
|---|---|---|
| AO-22 | N,N'-di-2-butil-1,4-fenilenediamine | Turbine and transformer oils, hydraulic fluids, waxes and fats |
| AO-24 | N,N'-di-2-butil-1,4-fenilenediamine | Low temperature oil |
| AO-29 | 2,6-di-tert-butil-4-methylphenol | Turbine and transformer oils, hydraulic fluids, waxes, fats and gasoline |
| AO-30 | 2,4-dimethyl-6-tert-butilphenol | Aircraft fuel |
| AO-31 | 2,4-dimethyl-6-tert-butilphenol | Aircraft fuel |
| AO-32 | 2,4-dimethyl-6-tert-butilphenol and 2,6-di-tert-butil-4-methylphenol | Aircraft fuel |
| AO-37 | 2,6-di-tert-butilphenol | widely used aircraft fuel |
Further reading
- Nick Lane Oxygen: The Molecule That Made the World (Oxford University Press, 2003) ISBN 0-19-860783-0
- Barry Halliwell and John M.C. Gutteridge Free Radicals in Biology and Medicine(Oxford University Press, 2007) ISBN 0-19-856869-X
- Jan Pokorny, Nelly Yanishlieva and Michael H. Gordon Antioxidants in Food: Practical Applications (CRC Press Inc., 2001) ISBN 0-8493-1222-1
Contenido relacionado
Maltase
Notoryctidae
Polypeptide