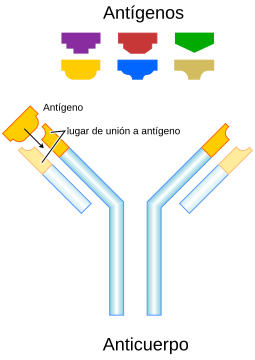
Antibody
The antibodies, (in science immunoglobulins Ig) are glycoproteins of the gamma globulin type. They can be found in a soluble form in the blood or other body fluids of vertebrates, having an identical form that acts as a membrane receptor on B lymphocytes and is used by the immune system to identify and neutralize foreign elements such as bacteria and viruses.
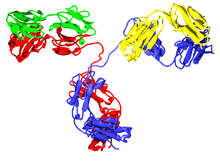
The typical antibody is made up of two basic structural units, each with two large heavy chains and two smaller light chains, forming, for example, monomers with one unit, dimers with two units, or pentamers with five. units. Antibodies are synthesized by a type of leukocyte called B lymphocyte. There are different types of antibody, isotypes, based on the form of heavy chain they have. Five different classes of isotypes are known in mammals that perform different functions, helping to direct the appropriate immune response for each different type of foreign body they encounter.
Although the general structure of all antibodies is very similar, a small region at the apex of the protein is extremely variable, allowing for the existence of millions of antibodies, each with a slightly different end. This part of the protein is known as the hypervariable region. Each of these variants can be attached to a "target" different, which is what is known as an antigen. This enormous diversity of antibodies allows the immune system to recognize an equally high diversity of antigens. The only part of the antigen recognized by the antibody is called an epitope. These epitopes bind with your antibody in a highly specific interaction called induced adaptation, which allows antibodies to identify and bind to only its unique antigen among the millions of different molecules that make up an organism.
Recognition of an antigen by an antibody marks it for attack by other parts of the immune system. Antibodies can also neutralize their targets directly, for example by binding to a portion of a pathogen necessary for it to cause an infection.
The large population of antibodies and their diversity is generated by random combinations of a set of gene segments encoding different antigen-binding sites (or paratopes), which subsequently undergo random mutations in this region of the antibody gene, resulting in even greater diversity. Antibody genes also rearrange in a process known as immunoglobulin class switching that swaps one heavy chain base for another, creating an antibody isotype different that maintains the variable region specific for the target antigen. This makes it possible for a single antibody to be used by different parts of the immune system. The production of antibodies is the main function of the humoral immune system. Antibodies, which are proteins that are part of the immune system, circulate in the blood. When they recognize foreign substances to the body, such as viruses and bacteria or their toxins, they neutralize them.
Antibodies, immunoglobulins and gammaglobulins
In general, as already stated in the introduction, it is considered that antibody and immunoglobulin are equivalent, the first term referring to the function, while the second alludes to the structure. The term gamma globulin derives from the electrophoretic properties of soluble immunoglobulins in serum, although some immunoglobulins migrate with alpha, beta, and even albumin.
The study of antibodies began in 1890 when Emil Adolf von Behring and Shibasaburo Kitasato described the activity of antibodies against diphtheria and tetanus toxin. Behring and Kitasato proposed the theory of humoral immunity, which established the existence of a mediator in the blood serum that could react with a foreign antigen, giving it the name of antibody. Their idea led Paul Ehrlich in 1897 to propose the theory of the side chain of the antigen-antibody interaction and to hypothesize that there were receptors (described as "side chains") on the surface of cells that could specifically bind toxins—in a side-chain interaction. key-lock type—and that this mating reaction was the trigger for the production of antibodies.
In 1904, following the idea of other researchers that antibodies were free in the blood, Almroth Wright suggested that soluble antibodies coated bacteria to signal them for phagocytosis and destruction in a process called opsonization.
In the 1920s, Michael Heidelberger and Oswald Avery discovered the nature of postulated antibodies by observing that antigens could be precipitated by them and showing that they were a type of protein.
In the late 1930s, John Marrack examined the biochemical properties of antigen-antibody binding. Then, in the 1940s, the next major advance took place, when Linus Pauling confirmed the proposed lock-and-key theory by Ehrlich showing that interactions between antibodies and antigens depended more on their shape than on their chemical composition. In 1948, Astrid Fagreaus discovered that B lymphocytes in their plasma cell form were responsible for the production of antibodies.
The following research works focused on the characterization of the molecular structure of antibodies:
- In the early 1960s, the main progress in this regard was made, with the discovery by Gerald M. Edelman and Joseph Gally of the light chain, and the understanding that this was identical to the Bence Jones protein described in 1845 by Henry Bence Jones. Edelman continued with the discovery that the antibodies were composed of light and heavy chains coupled with disulfuge links.
- By the same dates, Rodney Porter characterized the antibody binding regions (Fab) and the antibody tail (Fc) in the IgG type. Together, these scientists deduced the structure and complete sequence of amino acids of the IgG, so they received ex aequo the Nobel Prize in Physiology and Medicine in 1972.
- While most of these first studies were fixed on IgM and IgG, other immunoglobulin isotypes were identified in the 1960s: Thomas Tomasi discovered secreted antibodies (IgA) and David Rowe and John Fahey identified IgD, and IgE was identified by Kikishige Ishizaka and Teruki Ishizaka as an antibodies class.
- In 1975 César Milstein and Georges J. F. Köhler devise the method for the production of monoclonal antibodies. In 1976, genetic studies revealed the basis of the vast diversity of antibodies when the somatic recombination of immunoglobulin genes by Susumu Tonegawa was identified.
Forms of antibodies
Activated B lymphocytes differentiate into plasma cells, whose role is the production of soluble antibodies, or into memory B lymphocytes, which survive in the body for years to come to enable the immune system to remember the antigen and respond further to future exposures to the immunogenic agent. Antibodies are thus an essential product of the adaptive immune system that learn and remember responses to invading pathogens. Antibodies are found in two forms: in a soluble form secreted into the blood and other body fluids, and in a cell membrane-bound form that is anchored to the surface of a B lymphocyte.
Soluble form
Soluble antibodies are secreted by an activated B lymphocyte (in its plasma cell form) to bind to foreign substances and signal them for destruction by the rest of the immune system. They could also be called free antibodies until they bind to an antigen and end up as part of an antigen-antibody complex or as secreted antibodies.
In these soluble forms, additional molecules are attached to the immunoglobulins. In IgM, for example, we find a glycoprotein linked to the Constant Fraction by disulfide bridges of about 15 KD called the J chain. The IgA isotype is also joined by the so-called "secretion piece". It is a glycoprotein that is formed in epithelial cells and exocrine glands, and that subsequently binds to immunoglobulin to facilitate its secretion. (Pena, 1998)
Membrane-anchored shape
The membrane-anchored form of an antibody could be called surface immunoglobulin (sIg) or membrane immunoglobulin (mIg), which is not secreted: it is always associated to the cell membrane. It is part of the B lymphocyte receptor (BCR), which allows it to detect when a specific antigen is present in the body, triggering the activation of the B lymphocyte. The BCR is composed of IgD or IgM antibodies bound to the membrane surface and their associated Ig-α and Ig-β heterodimers that are capable of signaling transduction of antibody recognition to the cell. A typical human B lymphocyte has between 50,000 and 100,000 antibodies bound to the cell. their surface. Upon antigen binding, these clump together in large patches that can exceed 1 μm in diameter in lipid rafts that isolate BCRs (B cell receptors) from most other cell signaling receptors. These patches could improve the efficiency of the cellular immune response. In humans, the cell surface is free of other proteins around the B cell receptors at distances of a few thousand angstroms, thus reducing the influences that compete with their function, which even isolates the BCRs.
Isotypes, allotypes and idiotypes
Antibodies can come in several varieties known as isotypes or classes. In placental mammals there are five isotypes of antibodies known as IgA, IgD, IgE, IgG and IgM. They are named by the prefix "Ig" which stands for immunoglobulin and they differ in their biological properties, functional locations, and ability to recognize different types of antigens as shown in the table.
The isotype changes during the development and activation of B lymphocytes. Before B lymphocyte maturation, when they have not yet been exposed to their antigen, they are known as naïve B lymphocytes and only express the IgM isotype in its anchored to the cell surface. Lymphocytes begin to express both IgM and IgD when they reach maturity and are then ready to respond to their antigen. Activation of B lymphocytes follows the encounter and binding of its antigen, which stimulates the cell to that divides and differentiates into an antibody-producing cell called plasma. In this activated form, the B lymphocytes begin to secrete antibodies instead of anchoring them to the membrane. Some daughter cells of activated B lymphocytes undergo an isotypic switch, a mechanism that causes the production of antibodies in the IgM or IgD forms to be transmuted to the other types, IgE, IgA, or IgG, which play different roles in the immune system. But there is also an antibody that neutralizes and inactivates viruses, it is called "Neutralizing Antibody".
Allotypes
Allotype is understood as the small differences in the amino acid sequence in the constant region of the light and heavy chains of the antibodies produced by different individuals of a species, which are inherited in a Mendelian fashion (Peña, 1998).
In humans, 3 types of allotypic determinants have been described:
- In 1956 Grubb and Laurell discovered the Gm system in the IgG immunoglobulin class. This system revealed the various alotypes of heavy chains. It also allows to differentiate four subclasses in these molecules: IgG1, IgG2, IgG3 and IgG4 and are genetically determined.
- C. Ropartz and collaborators discovered in 1961 the system Km (called Inv at the beginning), located in the light chain Kappa. This alotype is present in all types of immunoglobulin.
- There is also the ISf system, located in the γ1 heavy chain of IgG1. The expression of this specificity increases with age, being 25% of the subjects before 20 years up to 60% after 70 years in the caucasoids.
- The alotypes defined by the Am system are located in the IgA, and more precisely in the α2 chains. There are two isotypes, α1 and α2, which characterize the Am1 and Am2 subclasses of the IgA. (Staff, 2003)
Idiotype
The idiotype is the proper epitope of a molecule belonging to a particular clone. This element forms part of or is very close to the antigen recognition site, and is located in the Fab variable portion. In other words, it is the paratope, or the nearby region of an immunoglobulin that can be recognized as an epitope by certain lymphocytes (Staff, 2003). According to the Jerne Theory, the formation of anti-idiotype antibodies would form a network (Jerne network) whose function would be to regulate the synthesis of new immunoglobulins. (Pena, 1998).
Structure
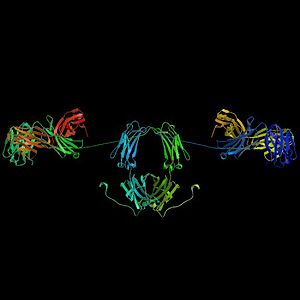
Antibodies are heavy (~150 kDa) globular plasma proteins, also known as immunoglobulins. They have sugar chains attached to one of their amino acid residues. In other words, antibodies are glycoproteins. The basic functional unit of each antibody is the immunoglobulin monomer, which contains a single Ig unit. Secreted antibodies can also be dimeric with two Ig units, as in the case of IgA, tetrameric with four Ig units as in the case of teleost IgM, or pentameric with five IgM units, as in the case of IgM. of mammals.
First works
The first investigations into the structure of antibodies were carried out by simple digestions with pepsin and papain by Rodney Robert Porter and Gerald M. Edelman, followed by electrophoresis. For this, both received the Nobel Prize in medicine in 1972. The figure of Alfred Nisonoff was also important:
- In the 1950s, Porter proceeds to make a mild digestion with papain, obtaining three fragments, two of which retained the specificity of antigen (Fab), while the third showed no union activity, while it could crystallize (Fc).
- In 1959, Edelman, using 2-Mercaptoethanel and urea, followed by electrophoresis, manages to isolate light and heavy chains by dissociating their disulfuro and non-covalent links.
- That same year, Porter identifies the components of light and heavy chains that were found in their fragments of papain and pepsin, and achieves their molecular weights.
- In 1960, Nisonoff showed that IgG's pepsin digestion produced a bivalent fragment, which is actually made up of two others, which he called F (ab')2.
Immunoglobulin Domains
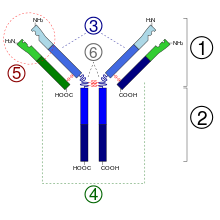
The Ig monomer is a "Y" consisting of two polypeptide chains; two identical heavy chains and two identical light chains connected by disulfide bonds. Each chain is made up of structural domains called Ig domains. These domains contain between 70 and 110 amino acids and are classified into different categories, for example variable (IgV) and constant (IgC) according to their size and function. They have an "immunoglobulin fold" characteristic in which two beta sheets generate a "sandwich" shape, held together by evolutionarily well-conserved interactions between cysteines and other charged amino acids.
Heavy Chain
There are five types of Ig in mammals named by Greek letters: α, δ, ε, γ, and μ. The type of heavy chain present defines the class (isotype) of the antibody. These chains are found in IgA, IgD, IgE, IgG, and IgM antibodies respectively. The different heavy chains differ in size and composition: α and γ contain approximately 450 amino acids, while μ and ε contain approximately 550 amino acids.
The γ, α, and δ heavy chains have a constant region composed of three tandem Ig framework domains and a hinge region to provide flexibility. The μ and ε heavy chains have a constant region composed by four immunoglobulin domains. The heavy chain variable region differs in antibodies produced by different B lymphocytes, but is identical for all antibodies produced by the same B lymphocyte or by its clonal lineage. The variable region of each heavy chain is approximately 110 amino acids and is composed of a single Ig domain.
Recently it has been possible to determine the in vivo topology of the heavy chain gene, Igh, this being one of the first studies in this field. The result is that the chromatin is arranged forming successive turns joined by "linkers", giving rise to shapes similar to a flower. The relative position of the different segments varies drastically throughout the development of the B lymphocyte, thus allowing a greater range of genomic interactions.
Light Chain
In mammals there are two types of light chain, called lambda (λ) and kappa (κ). A light chain contains two successive domains: a constant domain and a variable domain. The approximate length of the light chain is 211 to 217 amino acids. Each antibody contains two light chains that are always identical. Only one type of light chain, κ or λ, is present within the same antibody in mammals. Other types of light chains, such as the iota (ι) chain, are found in lower vertebrates such as chondrichthyans and teleosts.
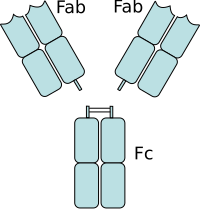
Fab and Fc Regions
Fab Region
Some parts of the antibody have unique functions. The 'ends of the Y', for example, contain the antigen-binding site and therefore recognize specific foreign elements. This region of the antibody is called the antigen-binding fragment or Fab region. It is composed of one constant and one variable domain from each of the antibody heavy and light chains. The paratope is made up of the heavy and light chain variable domains at the N-terminus of the antibody monomer.
Fc Region
The role of the "base of the Y" sector is to modulate the activity of the immune cell. This region is called Fc (from crystallizable fragment) and is composed of two or three constant domains from both heavy chains, depending on the class of the antibody. By binding to specific proteins the Fc region ensures that each antibody generates an immune response appropriate to a given antigen. The Fc region also binds to various cellular receptors such as the Fc receptor and other immune system molecules such as complement proteins. By doing this, it mediates different physiological effects including opsonization, cell lysis, and degranulation of mast cells, basophils, and eosinophils.
Function
Since antibodies occur freely in the bloodstream, they are said to be part of the humoral immune system. Circulating antibodies are produced by clonal lines of B lymphocytes that respond specifically to an antigen which may be a fragment of viral capsid protein, for example. Antibodies contribute to immunity in three different ways: they can prevent pathogens from entering cells or damage cells by binding to them (neutralization). They can stimulate the elimination of a pathogen by macrophages and other cells by coating the pathogen (opsonization) and can trigger direct destruction of the pathogen by stimulating other immune responses such as the complement pathway (lysis).
Activating the plugin
Antibodies that bind to the surface of antigens, for example, in a bacterium, attract the first components of the complement cascade through their Fc region and initiate activation of the "classical" complement. This kills the bacteria in two ways: First, the binding of complement molecules to the antibody marks the microbe for ingestion by phagocytes in a process called opsonization. These phagocytes are attracted by certain complement molecules. Second, some components of the complement system form a membrane attack complex to help the antibodies kill the bacteria through lysis. The most effective antibodies in the activation of the Complement System are those of the IgM type and those of the IgG subclass 1 and 3 type (IgG1 and IgG3).
Activation of effector cells
To combat pathogens that replicate on the outside of cells, antibodies bind to pathogens to assemble them together causing them to clump together. Since an antibody has at least two paratopes it can bind to more than one antigen by binding to identical epitopes carried on the surfaces of those antigens. By coating the pathogen, the antibodies stimulate effector functions against it in cells that recognize the Fc region.
Cells that recognize coated pathogens have Fc receptors that, as the name suggests, interact with the Fc region of IgA, IgG, and IgE antibodies. The coupling of a particular antibody with the Fc receptor of a certain cell triggers an effector function in it: phagocytes will carry out phagocytosis, mast cells and neutrophils will produce degranulation, natural killer cells will release cytokines and cytotoxic molecules that will finally kill the cell. the destruction of the invading microbe. Fc receptors are isotype specific, giving the immune system greater flexibility, affecting only the appropriate immune mechanism for different pathogens.
Diversity of Immunoglobulins
Virtually all microorganisms can trigger an antibody response. The recognition and successful eradication of very different types of the latter requires antibodies to possess enormous diversity. Their amino acid composition varies to allow them to interact with very different antigens. It has been estimated that humans generate about 10 billion different antibodies, each capable of binding to a different epitope. Although a huge repertoire of antibodies is generated different antibodies in the same individual, the number of genes available to make these proteins is limited. In vertebrates, different complex genetic mechanisms have evolved to allow B lymphocytes to generate this diversity from a relatively small number of antibody genes.
Domain variability
The region (locus) of the chromosome that codes for an antibody is large and contains several different genes for each domain of the antibody—the locus that contains the genes for heavy chains (IGH@) is found in humans on chromosome 14 and the loci containing the light chain lambda and kappa genes (IGL@ and IGK@) are located on chromosomes 22 and 2—. One of these domains is known as the 'variable domain', which is present in all light and heavy chains of antibodies, but may be different between the different antibodies generated by the various lines of B lymphocytes. between the variable domains are located in three loops known as hypervariable regions (HV-1, HV-2 and HV-3) or complementarity determining regions (CDR1, CDR2 and CDR3). The CDRs are maintained between the variable domains by framework regions. The heavy chain locus contains some 65 distinct variable domain genes, which differ in their CDRs. Combining these genes with several genes from other domains generates a large contingent of antibodies with a high degree of variability. This combination is called "V(D)J recombination, which we explain below.
V(D)J recombination
Somatic recombination of immunoglobulins, also known as V(D)J recombination, consists of the generation of a unique immunoglobulin variable region. The variable region of each heavy immunoglobulin is encoded by several parts, known as segments. These are known as variable (V), diversity (D), and joining (J) segments. V, D, and J segments are found in heavy chains. In light cells we only find segments V and J. There are multiple copies of all these segments organized in tandem in the mammalian genome. In the bone marrow, each developing B lymphocyte assembles the variable region of its immunoglobulin by randomly selecting and combining a V, a D, and a J segment (or a V and a J segment in the light chain). Since there are multiple slightly different copies for each genetic sequence of the segments, there would be different combinations that through this process generate a high number of paratopes and also different antigen specificities.
After the production of a functional immunoglobulin by a B lymphocyte during recombination, V(D)J will not be able to express any different variable region (this process is known as allelic exclusion). Thus, each B lymphocyte can only produce antibodies that contain a single type of variable chain.
Somatic hypermutation and affinity maturation
Another mechanism that generates antibody diversity occurs in mature B lymphocytes. Upon activation by antigen, B lymphocytes begin to proliferate rapidly. In these rapidly dividing cells, the genes encoding the heavy and light chain variable domains undergo a high rate of point mutation through a process called somatic hypermutation (SHM). This produces approximately one nucleotide change per variable gene and cell at each cell division. As a consequence, any daughter cell of a B cell line acquires a slight difference in the amino acid sequence of the variable domains of its antibody chains.
Somatic hypermutation serves to increase the diversity of the antibody pool and influences the binding affinity between antigen and antibody. Some point mutations will eventually produce antibodies that have weaker interactions (low affinity) with their antigen than the original antibody, while others will generate antibodies with a stronger interaction (high affinity). B cells expressing high affinity antibodies on their surface will receive a strong signal to survive during interactions with other cells, while those that express antibodies of low affinity will die by apoptosis. Thus, B cells that express antibodies with a higher affinity for their antigen will compete advantageously against those of lower affinity in their function and survival. The process of generating antibodies with progressively increased affinity is called affinity maturation. Affinity maturation occurs in mature B cells after V(D)J recombination and is dependent on the support they receive from helper T cells.
Class Change
Switching of the immunoglobulin class is a biological process that takes place after the activation of B lymphocytes, which allows the production of different classes of antibodies (IgA, IgE, or IgG). These classes are defined by the constant regions (C) of the immunoglobulin heavy chain. Initially, naïve B cells express only surface IgM and IgD with identical antibody-binding regions. Each isotype is adapted for a different function and therefore, upon activation, an antibody with an IgG, IgA or IgE effector is required for efficient antigen clearance. Class switching allows the progeny of a single B lymphocyte to produce antibodies of different isotypes. Only the constant region of the heavy chain of the antibody changes during class switching. The variable regions, and therefore the antigen specificity, remains unchanged. Thus, effectors are produced with the appropriate function for each antigen threat. Class switching is initiated by cytokines. The isotype generated depends on which cytokines are present in the environment of the B lymphocyte.
The process takes place in the heavy chain gene by a mechanism known as class switch recombination (CSR). This mechanism relies on conserved nucleotide sequences, called Switch or S Regions, that lie at a point in the DNA sequence before the constant region genes (except in the δ chain). The DNA strand is cleaved by the activity of certain enzymes at two specific S regions. The variable domain exon is spliced back together by a process called non-homologous end joining ("non-homologous end joining").; or NHEJ) to the chosen constant region (γ, α or ε). This process concludes by forming an immunoglobulin gene that encodes an antibody of a different isotype.
Gene conversion
Gene conversion is a non-reciprocal exchange, in which the donor sequence is unchanged, while the acceptor gene acquires a segment from the donor by homologous recombination. Although this mechanism for generating diversity in antibodies was known, it had not been given sufficient relevance until now. It is known to be very important in birds, which use in their light and heavy chains a large number of pseudogenes similar to D sequences, located at the beginning of the immunoglobulin chain gene sequence. Subsequently, these segments somatically change the single V region, and may also be subject to hypermutation. This mechanism, curiously, is also present in some mammals, such as rabbits.
Final stages of immunoglobulin synthesis
Once all the segments are regrouped, a single mRNA is produced, which is polyadenylated. This RNA leaves the nucleus, heading to the ribosomes of the rough endoplasmic reticulum, where its translation begins. Subsequently, their glycosylation occurs in the luminal part of the RER and the assembly, whose process is the following H+H → H2+L → H2L2. An exception is IgM, where a heavy chain first joins a light chain. Its final destination, as a receptor or to be secreted, depends on whether or not it has an added fragment of 19 amino acids in the C-terminal zone. This peptide is incorporated into the synthesis through a splicing process. Its presence determines a hydrophobic region capable of anchoring to the cell membrane (Peña, 1998).
Evolution of immunoglobulins
The development of complex organisms, with tissues and several cell lines, required the development of new molecules to ensure, on the one hand, that the cells adhered to others of the same colony and, on the other, the defense against possible parasitic intruders or pathogens. Three types of molecules, lectins, LLRs, and immunoglobulins, have been used throughout evolution in the development of immune systems. Their operational patterns are sometimes mixed to combine their properties, although there are few molecules that contain all three, such as the polycystic kidney disease (PKD1) gene.
Many studies provide important evidence that the immunoglobulin superfamily has representatives among bacteria and archaea, or at least those present in this group and those of eukaryotes could have a common ancestor, from which they evolved divergently. Thus, they have been attributed to this group of proteins "immunoglobulin-like" bacteria (BIg's) to the Ig Fc receptor in Streptococcus agalactiae, and endoglucanase C from Cellumonas fimi. Yersinia pseudotuberculosis or the Ligs (Leptospiral Ig-like) of various species of Leptospira. After the finding in Streptococcus such a protein was discovered in phage T4. On this occasion, it was highlighted that its role was related to cell adhesiveness.
Proteins with Ig domains are common in unicellular eukaryotes, and to some extent their structure is a conserved feature. An example of this would be the alpha agglutinins in Saccharomyces cerevisiae. These are molecules that measure cell adhesion and that have great homologies with the CD2-CD4 group in humans, whose role is partly similar, in the latter case involving the adhesion of T lymphocytes with antigen-presenting cells and cells. diana.
Multicellular Animals
However, it is in the most primitive groups of multicellular animals, the parazoa, where scientists try to find answers to the origin of the adaptive immune system. In this sense, several research works have been directed towards this group, and in especially towards a sponge considered to be a living fossil, Geodia cydonium and also Suberites domuncula. In this first are many of the types of proteins that are also involved in mammalian immunity. In particular, there are two types of the distinct immunoglobulin superfamily, those bound to receptor tyrosine kinases, and the non-enzymatic adhesion molecules of sponges. Interestingly, the corresponding domains already demonstrate polymorphism, and furthermore, although they fulfill roles that are simultaneously receptors and cell adhesion molecules, they are upregulated in grafting experiments.
In short, the immunoglobulin superfamily intervened in the emergence of multicellularity by maintaining the structural integrity of organisms, distinguishing what is self from what is foreign. This is because, thanks to their ability to generate modules, specifically bind to other proteins and form rods, as well as oligomerize and generate diversity by alternative splicing from limited genetic material, they become ideal for mediating cell adhesion. and as membrane surface receptors.
In the search for precedents of the adaptive immune system, we found several examples of proteins from the Ig superfamily in protostomes that play a role in immune defense, such as hemolin in silkworms, or the Dscam protein in Drosophila melanogaster, as well as fibrinogen-related proteins with Ig domains (FREP) in gastropods. Some of these proteins, which represent an innate barrier, can have soluble and membrane-anchored isoforms, and generate diversity by alternative splicing, and in areas of the molecule other than vertebrate variable chains.
Deuterostomes
Many of the elements of the adaptive immune system, including specialized cells, are already preconfigured in the most basal deuterostomous organisms. Work has been carried out on the sea urchin Strongylocentrotus purpuratus, finding a rich immune system with homologues of important immune and hematopoietic regulators of vertebrates, some of them critical. It is therefore speculated that the key evolutionary pressure for the development of the complex immune system in deuterostomes was not so much the threat of pathogens as the existence of a rich variety of symbiotic organisms, a circumstance that human beings themselves show in our intestinal flora.
Illustrating this point, 60% of echinoderm species have been found to associate with bacterial symbionts. In tunicates the increase in the complexity of the immune system continues. In the sea squirt Botryllus schlosseri, many proteins revealing a complex innate immune system and some immunoglobulin domain proteins were detected during mismatch grafting experiments. find a convincing homologue of RAG1, contiguous with a structure similar to RAG2. Later we will explain the importance of the latter. However, it is in cephalochordates that we find the first traces of our current immunoglobulins. Multiple studies have been carried out in the amphioxus Branchiostoma floridae, finding some curious proteins, called VCBPs (for V-type proteins that contain chitin-binding domains) with great homologies with the V (variable) regions of immunoglobulins, certainly involved in the immune response, but lacking in its variability. Crystallographic studies have shown that it is probably a molecule similar to the ancestor of the current vertebrate variable regions.
Some of the traits that identify a modern adaptive immune system are present in modern agnates, while others are absent. On the one hand, there are cells that already contain a large part of the molecular machinery of lymphocytes. This suggests an evolution of this cell type in the most basal vertebrates, and possibly in a protochordate. There are several Ig proteins with V-like domains, which even contain V and J regions, although they are encoded in a single exon and it is not rearrangeable. However, they do not possess an immune system like that of vertebrates, based on the classic soluble antibodies, membrane receptors, rearrangement, and RAG splicing. Instead, this function is taken over by a series of leucine repeat-rich proteins, which can even undergo complex recombination, resulting in variability comparable to that of antibodies (1014). This is an extraordinary example of parallel evolution.
Gnathostomes
All the authors reviewed in this article agree that the emergence of the modern immune system had to happen 500 million years ago, during the Cambrian explosion. They would probably do so within a context in which many shapes and combinations of protein modules would exist, many of which would disappear under selective pressures. In this sense, one of the questions that the previous section raises is that if the paleontological evidence indicates that the current jawed fish come from the agnaths, and these lack the same recombination system of the modern immune systems, surely there must have been a common ancestor, an ancestral ostracoderm that possessed both systems. According to this view, the V(D)J recombination system probably represents a convergent evolutionary development in a branch of ostracoderms that preceded the gnathostome line.
Regarding the classes of immunoglobulins, in fish we find analogues to the IgM class, as well as IgD, identified in many species of teleosts. There are also many exclusive ones, such as those containing the ζ and τ heavy chains. They are possibly isotypes that precede IgM in evolution. In the case of chondrichthyans we also find exclusive isotypes, in addition to IgM. These are IgW (IgX or IgNARC) and IgNAR.
The IgG type arises in amphibians and continues in reptiles, while the IgA type apparently arises in a common ancestor between birds and mammals. The IgE type seems to be exclusive to mammals (Peña, 1998).
Medical applications

Diagnosis of diseases
In many diagnoses, the detection of antibodies is common as a confirmation test of the pathology. For this, a serological test is performed. As examples, in biochemical tests for the diagnosis of diseases, the titer of antibodies against the Epstein-Barr virus or against Lyme disease is estimated. If these antibodies are not found, it means that the person is not infected or has been infected a long time ago and the B cells that make these antibodies are naturally reduced.
In clinical immunology, the levels of the different classes of immunoglobulins are assessed by nephelometry (or turbidimetry) to characterize the patient's antibody profile. For example, an observation of elevated titers of the different classes of immunoglobulins can be sometimes useful to determine the cause of liver damage through differential diagnosis. In this sense, a high IgA titer would indicate alcoholic cirrhosis; if IgM is elevated, viral hepatitis and primary biliary cirrhosis is suspected, while IgG is elevated in viral, autoimmune hepatitis, and cirrhosis.
Autoimmune diseases can be diagnosed by antibodies that bind to epitopes of the organism itself; many of them can be detected through blood tests. An example would be the case of antibodies directed against erythrocyte surface antigens in immune-mediated hemolytic anemia, which are detected by the Coombs test. This test is also used to screen for antibodies in the preparation of blood transfusions. blood and also in women in the prenatal period.
In practice, there are many immunodiagnostic methods based on the detection of antigen-antibody complexes that are used in the diagnosis of infectious diseases, for example ELISA, immunofluorescence, Western blot, immunodiffusion and immunoelectrophoresis.
Therapeutic Treatments
Monoclonal antibody therapy is used in the treatment of diseases such as rheumatoid arthritis, multiple sclerosis, psoriasis, and many forms of cancer, including non-Hodgkin lymphoma, colorectal cancer, head and neck cancer, and breast cancer. Some immunodeficiencies, such as X-linked agammaglobulinemia and hypogammaglobulinemia, consist of a partial or complete lack of antibodies. These diseases are sometimes treated by inducing a short-term immunity called passive immunity. This is acquired through the infusion of "prefabricated" in the form of human or animal serum, intravenous immunoglobulin, or monoclonal antibodies in the affected individual.
Prenatal Therapy
The so-called Rho (D) Immunoglobulins or anti-RhD immunoglobulins are specific for the human Rhesus D antigen, also known as Rhesus factor. Several trademarks are known for these anti-RhD antibodies, such as RhoGAM, BayRHo-D, Gamulin Rh, HypRho-D, and WinRho SDF. Rhesus factor is an antigen found on red blood cells. Rhesus-positive (Rh+) individuals display this antibody in the glycocalyx of their erythrocytes, whereas (Rh–) individuals lack it.
During normal birth, fetal blood can pass to the mother from birth trauma or pregnancy complications. In the case of Rh incompatibility between mother and child, the consequent mixing of blood can sensitize an Rh- mother to the Rh antigen of the child, placing subsequent pregnancies at risk of erythroblastosis fetalis. administered as part of prenatal treatment to prevent sensitization that might take place to avoid it. Treating the mother with anti-RhD antibodies before and immediately after trauma and delivery destroys the Rh antigen of the fetus in the mother's body. An important issue is that this happens before the antigen can stimulate maternal B cells that might later "remember" the cells. the Rh antigen generating memory B lymphocytes. Therefore, her humoral immune system will not make anti-Rh antibodies and will not attack the Rhesus antigens of her current or future baby.
Applications in scientific research
In research, purified antibodies are used in many applications. They are very common to identify and localize intracellular and extracellular proteins. Antibodies are used in flow cytometry to differentiate cell types by the proteins they express; different cell types also express different combinations of cluster of differentiation (CD) molecules on their surface and produce different intracellular, extracellular, and excretable proteins. They are also used in immunoprecipitation to separate proteins and anything that is bound to them (co -immunoprecipitation) of other molecules in a cell lysate, in Western blot analysis to identify proteins separated by electrophoresis, and in immunohistochemistry or immunofluorescence to examine protein expression in tissue sections or localize proteins within cells with the aid of a microscope. Proteins can also be detected and quantified with antibodies, using ELISA and ELISPOT techniques.
Antibody variants in medicine and research
Sometimes you need to produce specific antibodies. Injecting an antigen into a mammal, such as a mouse, rat, or rabbit if a small amount is required; Goat, sheep or horse if large quantities are required. The blood isolated from these animals contains polyclonal antibodies—multiple antibodies that bind to the same antigen—in the blood serum, which is called antiserum. Antigens can also be injected into chickens and the IgY polyclonal antibodies produced can be collected in the egg yolk. therapeutic applications in which it is desired to block or detect very specific markers. For this reason, antibody technology has generated some variants, among which the following stand out:
- Monoclonal antibodies
- If you want to get specific antibodies for a single epitope of an antigen, secret antibodies lymphocytes are isolated from an animal's antibodies and are immortalized by merging them with a cancerous cell line. The merged cells are called hybridomas and will continue to grow and secrete antibody in the crop. Individual hybridoma cells are isolated by dilution cloning to generate clones that all produce the same antibody. These antibodies are called monoclonal antibodies.
Generated monoclonal and polyclonal antibodies can be purified using protein A/G or antigen affinity chromatography.
- Simple chain antibodies
- It is possible to artificially generate an antibody that has only the variable regions of the light and heavy chain, coupled with a small peptide or a single amino acid. In this case we will have simple chain antibodies or scFv's. They are currently applied in techniques such as flow cytometry or immunohistochemicals.
- Abzimas
- Most antibodies are differentiated from other proteins because they do not present enzyme calysis in their function, so they are traditionally considered proteins for recognition of molecular surfaces. However, in the 1990s and early 21st century, various immunology studies found antibodies with catalytic properties. These antibodies have received the name of abzimas. It is possible to find them in low amounts in the serum of healthy people. An example of the existence of abzimas in the human body was the detection of abzimas against DNA in breast milk. Among some of these detected catalytic activities are those of inspecific and amylolytic peptides (starch degradation). On the other hand, there has been an increase in the level of abzymes in autoimmune diseases. However, they are usually manufactured artificially by generating antibodies against the intermediate compound of a reaction for which an enzyme is wanted. Sometimes they could have therapeutic and industrial applications.
- Nanoantibodies
There are proposals for the therapeutic use of camelid monoclonal antibodies, also called nanoantibodies. These are exceptional in the animal kingdom, given their small size, since they are composed only of two heavy chains. Such peculiarities would allow them to access cellular locations and antigens inaccessible to normal antibodies, as well as being possible for oral administration.
- Faboterápicos
To obtain antidotes against venoms from animal bites such as snakes or arthropods, antisera are made using crude serum or serum highly enriched in immunoglobulins. These procedures produced a large number of allergic reactions, such as anaphylaxis or serum sickness. To avoid this, in the 40s and 50s proteolysis studies were carried out to minimize the part of the molecule involved in the neutralization of the venom. Finally, it was found that the F(ab')2 fragment, resulting from pepsin digestion of the antibodies, which lacks the effector zones of the molecule, can also neutralize poisons. Professor Alejandro Alagón Cano proposed the name fabotherapy for this therapeutic approach, observing a much lower incidence of adverse reactions to serum, as well as a better reach of the extravascular compartment.
Contenido relacionado
Harvard Medical School
DNA polymerase
Ornithology