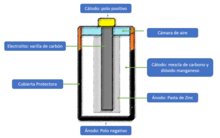
Anode
The anode is an electrode where an oxidation reaction occurs, through which a material, by losing electrons, increases its oxidation state.
Etymology
Faraday (in series VII of the Experimental Investigations on Electricity) used the term for the first time, with the meaning of "upward path" or "of playing or entrance" [from the Greek ανά (aná): upwards, and οδός (odós): path], but referring exclusively to the electrolyte of an electrochemical cell.
Its connection to the positive pole of the corresponding generator implies transit of the electric current through the external circuit from the positive to the negative pole; that is, carried by positive charges.
| Galvanic cell | Electrolytic cell | |
|---|---|---|
| Type of conversion | Chemical energy → Electric energy | Electrical energy → Chemical energy |
| Positive electrode | Method (reduction) | Anode (oxidation). |
| Negative electrode | Anode (oxidation) | Method (reduction) |
|
The polarity of the anode, positive or negative, depends on the type of device and, sometimes, on the way it operates, since it is established according to the direction of the electric current, according to the universal definition of this phenomenon. Consequently:
|
It would seem logical to define the direction of electric current as the direction of movement of free charges. However, if the conductor is not metallic, there are also positive charges moving through the external conductor (the electrolyte of the cell), and whatever the agreed direction, there would be charges moving in opposite directions. Therefore, the agreement is adopted that its definition of the direction of the current is "the route through the positive charges (cations), and that consequently it is from positive to negative: anode → cathode".
In the cases of thermoionic valves, electrical sources, batteries, etc., the anode is the electrode or terminal with the highest potential. In a redox reaction it corresponds to the element that will be oxidized.
Thermionic valves
Thermionic valves anode receives most of the electrons emitted by the cathode. When it comes to amplifier tubes (triodes, tetrodes or pentodes) and especially if they are power tubes, this electrode generates the heat that occurs in the tube, which must be dissipated. There are two procedures for this, depending on the case:
- Great power. The anode is thermally attached to a dissipator outside the valve, which is refrigerated by air circulation, steam, oil, etc.
- Lower power. In this case the anodes are large, with wide surface to the outside, so they dissipate by radiation.
The origin of this heat lies in the energy that the electrons acquire when subjected to the difference in voltage between the two electrodes. When they hit the anode, they give up their energy, which is dissipated by heat.
Special anodes
Certain valves contain special anodes, as in the case of X-ray tubes (Röntgen tubes). X rays are generated by striking high-energy electrons against the anode atoms, commonly tungsten or molybdenum. The energy of these electrons, in addition to being emitted in the form of X-rays, greatly heats the anode. For this reason it is provided with heat sinks, by air, oil or water. They are usually rotating, with a motor that drives them to distribute the incidence of electrons over a vast surface.
In cathode ray tubes the anode surrounds the screen. It is connected to it by a thin layer of aluminum deposited on the tube.
The anode of magnetrons usually takes the form of resonant cavities at the operating frequency.
In some hot water or heat transfer fluid accumulators for solar thermal solar panels, a sacrificial anode is installed in the accumulator when electrical anodization is not available. This is used to force the sacrificial anode to corrode at the expense of the other elements (cathodic protection), to allow the equipment to withstand the galvanic cell that makes the system in its operating conditions (metals and compounds of elements of different conductivity, in water with temperature fluctuations). This is a very important part of preventive maintenance and promotes the durability of the equipment and the system, since this same anodic corrosion also increases the deposition of lime (and other salts present in the water) in the circuit.
Contenido relacionado
Acid dissociation constant
Triglyceride
Sodium