Ammonium
Ammonium is a positively charged polyatomic cation, with the chemical formula NH4+. It has a molecular weight of 18.04 and is formed by protonation of ammonia (NH3).
H++:NH3 ammonia NH4+ ionDamn.{displaystyle mathrm {H^{+} +underbrace {mathrm {:!NH_{3}} } _{textrm {amonym}}rightleftharpoons underbrace {mathrm {NH_{4}{textrm}{textrm {textrm}{textrm}{textr}{textr}{The resulting ion has a pKa of 9.25. The names ammonium and aminium are also names for protonated or positively charged substituted amines, and quaternary ammonium cations N+R4, where one or more hydrogen atoms are replaced by alkyl groups (which can be symbolized as R).
Chemistry
Ammonia is a weak base: it reacts with Brønsted acids (proton donors) to produce the ammonium ion. When ammonia is dissolved in water, a small amount of it reacts with hydronium ions in the water to produce ammonium ions. The resulting ammonium ion is a comparatively strong conjugate acid, and reacts with any base, regenerating the neutral ammonia molecule. In aqueous solution, the degree to which ammonia forms ammonium ions depends on the pH of the solution and the concentration of ammonia.
- H++:NH3 ammonia NH4+ ionDamn.{displaystyle mathrm {H^{+} +underbrace {mathrm {:!NH_{3}} } _{textrm {amonym}}rightleftharpoons underbrace {mathrm {NH_{4}{textrm}{textrm {textrm}{textrm}{textr}{textr}{
The lone electron pair on nitrogen (N) in ammonia is represented as a pair of dots. This pair of electrons forms the bond with the hydrogen cation H+.
In the ammonium ion, the nitrogen atom forms four covalent bonds, instead of three as in ammonia, forming a structure that is isoelectronic to the methane molecule and, consequently, is energetically favorable.
The formation of ammonium compounds can also occur in the vapor phase; For example, when ammonia vapors come into contact with hydrogen chloride vapors, a white cloud of ammonium chloride is formed, eventually depositing as a thin layer of solid on surfaces. Ammonium cations resemble alkali metals such as Na+ or K+ and can be found in salts such as ammonium bicarbonate, ammonium chloride, and ammonium nitrate. ammonium. The simplest ammonium salts are highly soluble in water.
Reduction of the ammonium cation releases ammonia gas and hydrogen:
- 2NH4++2eΔ Δ 2NH3+H2{displaystyle 2{NH_{4}}}^{+}+2elongrightarrow 2NH_{3}+H_{2}}}
Ammonium radicals can dissolve in mercury to form an amalgam. It can practically be carried out by electrolysis of an ammonium solution with a mercury electrode. This amalgam decomposes spontaneously to produce ammonia and hydrogen.
Organic Radical Groups
Depending on the degree of substitution of the hydrogen by alkyl groups, the group can be called an primary, secondary, tertiary, or tertiary ammonium cation. quaternary. They exist in equilibrium with their respective substituted amine, depending on the pH.
Only quaternary ammonium cations are permanently charged. These cations, eg. tetra-n-butylammonium cation are sometimes used to replace sodium or potassium ions and increase the overall solubility of compounds in organic solvents, based on HSAB principles. Quaternary ammonium salts are frequently used as phase transfer catalysts for the same reason.
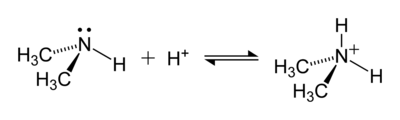
An example of a reaction that forms an ammonium ion is that between dimethylamine, (CH3)2NH, with an acid to produce the dimethylamino cation, (CH3)2NH2+:
Biology
Ammonium ions are a toxic waste product of metabolism in animals. In fish and aquatic invertebrates, it is excreted directly into the water. In mammals, sharks, and amphibians, it is converted in the urea cycle to urea, because it is less toxic and can be stored more efficiently. In terrestrial birds, reptiles, and snakes, metabolic ammonia is converted to uric acid, which is solid and can be excreted with minimal water loss.
Ammonia is toxic to humans in high concentrations, and can cause damage to the lining of the lungs, or alkaline burns.
Contenido relacionado
Olga Ladyzhenskaya
Cx
Annex: Manned missions to space by program