Amine
In organic chemistry, amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (NH
3), where one or more hydrogen atoms of the ammonia molecule are replaced by other substituents or radicals such as an alkyl or aryl group (these may be called alkylamines and arylamines, respectively; amines in which both types of substituents are attached to a nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganics derived from ammonia are also called amines, such as monochloramine (NClH
2).
The substituent −NH
2 is called the amino group.
Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure R−CO−NR′R″, are called amides and have different chemical properties. amine properties.
As one, two, or three hydrogens are substituted, amines are primary, secondary, or tertiary, respectively.
| Amonia | Primary Amine | Secondary | Amina terciaria |
|---|---|---|---|
 | 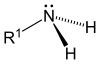 |  | 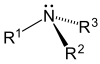 |
Classification of amines
Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. aromatic amines have the nitrogen atom connected to an aromatic ring.
Amines, alkyl and aryl alike, are organized into three subcategories (see table) based on the number of carbon atoms adjacent to nitrogen (how many hydrogen atoms in the ammonia molecule are replaced by hydrocarbon groups):
- Primary aminas (1°): Primary amines arise when one of the three hydrogen atoms in the ammonia is replaced by an alkylo or aromatic group. Important primary alkylic amines include metilamine, most amino acids and tris damping agent, while primary amines include aniline.
- Secondary aminas (2°): Secondary amines have two organic substitutes (alkylo, arilo or both) attached to nitrogen together with a hydrogen. Some important representatives are dimethylamine, while an example of amine aromatics would be diphenylamine.
- Tertiary mines (3°)- In tertiary amines, nitrogen has three organic substitutes. Some examples are trimetilamine, which has a distinctive smell of fish, and EDTA.
A fourth subcategory is determined by the connectivity of the substituents attached to nitrogen:
- Cycling aminas- Cyclical amines are secondary or tertiary amines. Examples of clicked amines include the ring of 3 aziridina members and the ring of six piperidina members. La N- methylpiperidine and N-phenilpiperidine are examples of tertiary clicas amines.
It is also possible to have four organic substituents on nitrogen. These species are not amines but are quaternary ammonium cations and have a charged nitrogen center. There are sales of quaternary ammonium with many types of anions.
Amines are simple when the alkyl groups are the same and mixed if they are different.
Amines are very polar compounds. Primary and secondary amines can form hydrogen bonds. Pure tertiary amines cannot form hydrogen bonds, however they can accept hydrogen bonds with molecules that have O-H or N-H bonds. Since nitrogen is less electronegative than oxygen, the N-H bond is less polar than the O-H bond. Therefore, amines form weaker hydrogen bonds than alcohols of similar molecular weights.
Primary and secondary amines have boiling points lower than those of alcohols, but higher than those of ethers of similar molecular weight. Tertiary amines, without hydrogen bonding, have lower boiling points than primary and secondary amines of similar molecular weights.
Amine nomenclature
Amines are classified according to the number of hydrogen atoms in ammonia that are replaced by organic groups. Those with only one group are called primary amines, those with two are called secondary amines, and those with three are called tertiary amines.
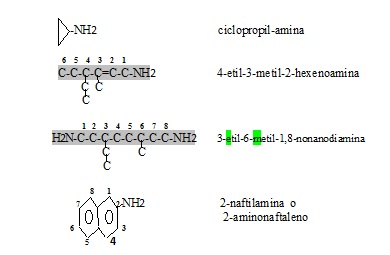
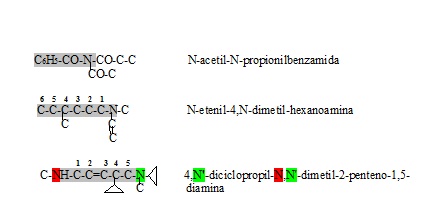
Simple amines are named by listing the groups that replace the hydrogen atoms in ammonia and ending with amine. If there are several identical substituent groups or radicals, the prefixes di or tri are used. When it comes to different groups, they are named in alphabetical order (ethyl before methyl, or butyl before propyl, regardless of size) and ending with the ending amine.
Examples:
| Compound | Names |
|---|---|
| CH3-NH2 | Metilamina |
| CH3-NH-CH3 | Dimetilamine |
| CH3-CH2-NH-CH2-CH2-CH3 | Etilpropylamine |
| CH3 日本語 N-CH3 日本語 CH3 | Trimetilamina |
| CH3 日本語 N-CH2-CH2-CH3 日本語 CH2-CH3 | Etilmetilpropylamine |
Rules for naming amines
- 1.1. The main chain that the amino group has is identified and is listed by the carbon to which the amino group is attached. If there are two or more amino groups, they are named from the end that gives rise to the minor prefixes locators (position) of the substitutes and are named in alphabetical order with the word amina.
- 1.2. When there are complex radicals (not radical alkylo) replacing the hydrogen of the amino group, the letter N is used by each substitute and the compound is appointed.
- 1.3. If the amino group is found as a substitute next to another more important functional group and in the case of existing several in a chain prefixes are used as (amino, metil, aminomethyl). The amino group must remain in the lowest position.
- 1.4. When several nitrogen atoms are part of the main chain it is usually listed seeing that their position is the lowest possible and names with the vocable aza
Structure
Alkyl Amines
Alkyl amines are characterized by having tetrahedral nitrogen centers. The angles C-N-C and C-N-H approximate the idealized angle of 109°. The C-N distances are slightly shorter than the C-C distances. The energy barrier for nitrogen inversion of the stereocenter is approximately 7 kcal/mol for a trialkylamine. Interconversion has been compared to the inversion of an open umbrella in a strong wind.
NHRR' and NRR′R″ are chiral: the nitrogen center bears four substituents counting the lone pair. Due to the low investment barrier, amines of the type NHRR' they cannot be obtained with optical purity. In the case of chiral tertiary amines, the NRR′R″ can only be resolved when the R, R' and R″ are constrained in cyclic structures like the N-substituted aziridines (quaternary ammonium salts are resolvable).
Aromatic amines
In aromatic amines ("anilines"), the nitrogen is usually nearly planar due to conjugation of the lone pair to the aryl substituent. The C-N distance is correspondingly shorter. In aniline, the C-N distance is the same as the C-C distances.
Physical and physiological properties
Aliphatic amines, such as monomethylamine, dimethylamine, and trimethylamine, as well as ethylamine, are gases at room temperature. Many other homologous compounds are liquids and some even higher homologous amines such as decylamine are solid at room temperature.
The simplest aromatic amine aniline is liquid. Many substituted anilines and other aromatic amines with multiple aromatic ring systems, such as naphthylamines, are solid.
Due to their polarity and basicity, amines are more soluble in water than hydrocarbons with the same number of C atoms. The solubility of aliphatic amines in water decreases with increasing alkyl chain length. Aromatic amines are not soluble in water. Liquid aliphatic and aromatic primary and secondary amines associate by hydrogen bonding. This means that, like alcohols, the boiling points are higher than those of analogous hydrocarbons.
Gasous aliphatic amines irritate the mucous membranes of the eyes and respiratory tract. Wetting the skin with liquid alkylamines also causes burns. Inhalation poisoning from higher concentrations can cause an increase in blood pressure and short-term convulsions. Aromatic amines are not irritating due to their lower basicity and volatility, but they are significantly more toxic than aliphatic amines, such as aniline.
Gas aliphatic amines have an odor similar to ammonia but with an additional "fishy, putrid" odor. The higher homologues and the aromatic or heterocyclic amines also have odors that are perceived as unpleasant by humans, for example, feces (indole, skatole), or decaying meat (cadaverine, putrescine), urine, or old fish (methyl, ethyl and trimethylamine). These compounds can appear as intermediate or final products during the anaerobic process. degradation of biological material, especially proteins, or by decarboxylation of amino acids. The characteristic odor of spermine is due to spermine - a linear polyamine - with two primary and two secondary amino groups.
On the other hand, many drugs also belong to the group of amines, especially often to the subgroup of heterocyclic amines, such as atropine, amphetamine, quinine, codeine and caffeine, but also drugs such as methamphetamine, cocaine, nicotine.
Detection of amines
- To detect nitrogen in an organic compound, a sodium digestion of the substance may be performed to examine. In the neutralized digestion solution, nitrogen can be detected as cyanide with the Lassaigne test as Prussian blue or, if the substance also contained sulfur, such as thiocyanate with ferrous chloride. However, this evidence is not specific to amines, but only indicates that the substance of analysis contained nitrogen.
- Amines can often be identified by their characteristic odor acre or unpleasant (such as ammonia to fish). But that's not enough as proof.
- To determine the degree of substitution of amine, i.e., if a primary, secondary or tertiary amine is present, the separation of Hinsberg is carried out. Here, amine becomes p-amide of toluenosulfonic acid:
Tertiary amines and quaternary ammonium salts do not form sulfonamides,
Secondary amines form sulfonamides that are not soluble in alkali.
Primary amines form sulfonamides that are soluble in alkali.
- An unknown amine is clearly identified either mass spectrometry or by an appropriate derivative whose characteristic fusion point is determined:
- Primary and secondary aminas: Sulfonamide already obtained from the separation of Hinsberg is suitable as derivative (see above).
- Tertiary aminas: Here is recommended the precipitation of picrato.
Primary, secondary and tertiary amines can be separated chromatographically without derivatization by HPLC. Detection and quantification is performed with a mass selective detector (HPLC/MS). For the unequivocal determination of amines of the same molar mass (eg diethylamine and butylamine), the use of standard substances for calibration is recommended.
Biological activity
Amines are ubiquitous in biology. The breakdown of amino acids releases amines, as is the case with decaying fish, which smells like trimethylamine. Many neurotransmitters are amines, such as epinephrine, norepinephrine, dopamine, serotonin, and histamine. Protonated Amino groups (-NH
3) are the most common positively charged elements in proteins, specifically in the amino acid lysine. The anionic polymer DNA is usually bound to various amine-rich proteins. In addition, the terminally charged primary ammonium of lysine forms salt bridges with the carboxylate groups of other amino acids in polypeptides, constituting one of the main influences on the three-dimensional structures of proteins.
Use
Aromatic amines are used to make azo dyes. Amines are building blocks of agrochemicals and pharmaceuticals, as well as surfactants, coatings, and lubricants. In the field of foundry technology, amines are used as catalysts to speed up the curing process of binders in molding sand during core production using the cold box process. Amines and diamines are also used as catalysts for the production and crosslinking of polyurethanes. Their buffering effect is used when used as corrosion inhibitors in aqueous systems. Another important field of application of amines is the purification of gases in refineries and power plants.
Contenido relacionado
Ammophila (plant)
Aeluropus
Alfaroa