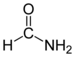
Amide
An amide is a compound that is formed conceptually or chemically by replacing the hydroxyl of an oxacid with an amino substituent. In organic chemistry, it is called par excellence as "amide" to amides of carboxylic acids (strictly, carboxamide). It can be considered as a derivative of a carboxylic acid by substituting the —OH group of the acid for a —NH2, —NHR or —NRR' (called an amino group). For this reason, its functional group is of the RCONR'R'' type, with CO being a carbonyl, N a nitrogen atom, and R, R' and R'' organic radicals or hydrogen atoms:
When the amide group is not the main one, it is named using the prefix carbamoil:
CH3-CH2-CH(CONH2)-CH2-CH 2-COOH → 4-carbamoylhexanoic acid.
All amides except the first in the series are solid at room temperature and have high boiling points, higher than those of the corresponding acids. They have excellent solvent properties and are very weak bases. One of the main methods of obtaining these compounds consists of reacting ammonia (or primary or secondary amines) with esters. Amides are common in nature, and one of the best known is urea, a diamide that does not contain hydrocarbons. Proteins and peptides are made up of amides. An example of a long chain polyamide is nylon. Amides are also widely used in the pharmaceutical industry.
Polyamides
Carbonic acid amide is called urea and its derivatives are the ureido functional group.
Diacylamines are denominated as imide functional group and are analogous to carboxylic anhydrides, for example carboxyl
Polyamides are compounds that contain amide groups. Some are synthetic, such as nylon, but they are also found in nature, in proteins, formed from amino acids by reaction of a carboxyl group of an amino acid. with one amino group from another. In proteins, the amide group is called a peptide bond.
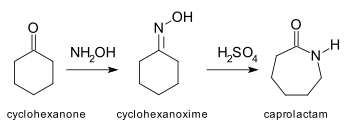
Nylon is a polyamide due to the characteristic amide groups in the main chain of its formulation. For example, nylon 6 is obtained by polymerization of ε-caprolactam.
Certain nylon-type polyamides are polyamide-6, polyamide-11, polyamide-12, polyamide-9,6, polyamide-6,9, polyamide-6,10, and polyamide-6, 12. As an example of non-linear polyamides, the condensation products of dimerized acids of vegetable oils with amines may be mentioned.
Peptides, including proteins like silk, which nylon replaced, are also polyamides. These amide groups are highly polar and can be linked together by hydrogen bonding. Because of this, and because the nylon chain is so regular and symmetrical, nylons are often crystalline, making excellent fibers.
Synthesis of amides
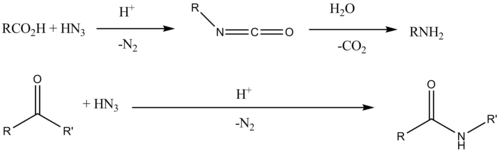
The synthesis of amides can be carried out by various methods. The simplest method is the condensation of a carboxylic acid with an amine. This reaction is thermodynamically favorable in general, but has a high activation energy, mainly due to the first deprotonation of the carboxylic acid and the protonation of the amine, which forms a stable product, the amino carboxylate. This reduces the reactivity. Also, high temperatures are required.
- RCO2H + R′R′NH
 RCO−
RCO−
2 + R′R′NH+
2 RC(O)NR′R′ + H2O
RC(O)NR′R′ + H2O
Many methods are known to drive the equilibrium to the right. For the most part, these reactions involve "activating" the carboxylic acid by first making it a better electrophile; such as esters, acid chlorides (Schotten-Baumann reaction) or anhydrides (Lumière-Barbier method). Conventional methods in peptide synthesis use coupling agents such as HATU, hydroxybenzothiazole (HOBt) or PyBOP. Recently, novel boron-based reagents have emerged for CO-N bond formation, including the use of acid catalysts. 2-iodophenylboronic acid, MIBA, and tris(2,2,2-trifluoroethyl) borate.
Reactions of amides
The main reactions of amides are:
- Acid or basic hydrolysis: The amide is hydrolyzed in the basic medium by forming a metal carboxylate or in the acid medium forming a carboxylic acid.
- Dehydration: In the presence of a dehydrant such as tionyl chloride or phosphorus pentoxide a nitrile is produced.
- Reduction: Amids can be reduced with lithium and aluminium amines.
- Hofmann's Transposition: In the presence of a halogen in the basic medium, a complex reaction is produced that allows to obtain amine with a less carbon in its main chain.
Example of amide
- Acrylamide is used in different applications, although it is best known for being probably carcinogenic and being present in enough foods as it is formed by natural processes when cooking them.
- They are a source of energy for the human body.
- They can be vitamins in the body or painkillers.
Importance and uses
Amides are common in nature and are found in substances such as amino acids, proteins, DNA and RNA, hormones, and vitamins.
Urea is used for the excretion of ammonia (NH3) in humans and mammals. It is also widely used in the pharmaceutical industry and in the nylon industry.
Contenido relacionado
Lactose
Ethanol
Saccharose