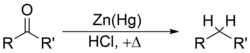Alkane
The alkanes are hydrocarbons, that is, they are compounds that only contain carbon and hydrogen atoms. The general formula for aliphatic (straight-chain) alkanes is CnH2n+2, and for cycloalkanes is CnH2n. They are also called saturated hydrocarbons, since they lack double or triple bonds and, therefore, all their carbons present sp3 hybridization. In addition, they lack functional groups.
Alkaline (primary) alkanes
Aliphatic alkanes can be straight-chain or branched, with the ratio of carbon and hydrogen being CnH2n+2, where "n" represents the number of carbon atoms in the molecule. Its reactivity is very low compared to other organic compounds. All the bonds of alkanes are simple (that is, of the sigma type), that is, they are covalent that share a pair of electrons in an s orbital, for which reason the structure of an alkane would be as follows: shape, where each line or bar represents a covalent bond where a pair of electrons is equally shared between the bonded atoms.
The simplest alkane is methane with a single carbon atom. Other known alkanes are ethane, propane and butane with two, three and four carbon atoms respectively. Starting with five carbons, the names are derived according to the number of carbon atoms in the molecule.
| C | Name | Formula | Model |
|---|---|---|---|
| 1 | Methane | CH4 | |
| 2 | Etano | C2H6 | |
| 3 | Propane | C3H8 | |
| 4 | Butano | C4H10 | |
| 5 | Pentano | C5H12 | |
| 6 | Hexano | C6H14 | |
| 7 | Heptano | C7H16 | |
| 8 | Octan | C8H18 | |
| 9 | Nonano | C9H20 | |
| 10 | Dean | C10H22 | |
| 11 | A dean | C11H24 | |
| 12 | Dodean | C12H26 |
Polyethylene (PE) is chemically the simplest polymer represented with its repeating unit (CH2-CH2) n. It is obtained from the polymerization of ethylene. Ultra-high molecular weight polyethylene (UHMWPE, UHMW) is an extremely long unbranched linear chain polymer, with a molecular mass generally between 3.5 and 7.5 million Daltons.
For the nomenclature of acyclic alkanes, see Nomenclature of acyclic hydrocarbons.
Cycloalkanes
Cyclic alkanes or cycloalkanes are, as their name suggests, cyclic chain hydrocarbons. In them the relation C/H is CnH2n. Their physical characteristics are similar to those of non-cyclic alkanes, but their chemical characteristics differ significantly, especially those with shorter chains, which have properties more similar to those of alkynes.
For details of the nomenclature of cycloalkanes, see Nomenclature of Monocyclic Hydrocarbons, Nomenclature of Bridged Polycyclic Hydrocarbons, Spiro Compound, Fusion Nomenclature System, and Nomenclature of Cycle Groupings
Abundance
Abundance of alkanes in the universe
Alkanes are an important part of the atmospheres of the outer planets, such as Jupiter (0.01% methane, 0.0002% ethane), Saturn (0.2% methane, 0.0005% ethane), Uranus (1.99% methane, 0.00025% ethane), and Neptune (1.5% methane, 1.5 ppm ethane). Titan, a satellite of Saturn, was studied by the Huygens space probe, and found that liquid methane rains from Titan's atmosphere. A methane-spewing volcano was also observed on Titan, and this volcanism is believed to be a significant source of methane. in the atmosphere. It also appears that there are methane/ethane lakes near Titan's northern polar regions, as discovered by the Cassini spacecraft's radar imaging system. Methane and ethane have also been detected in the tail of Comet Hyakutake. Chemical analysis showed that the abundance of ethane and methane are approximately equal, which is thought to imply that ices formed in interstellar space, far from the Sun, could have evaporated unevenly due to the different volatility of these molecules. Alkanes have also been detected in meteorites such as carbonaceous chondrites.
Abundance of alkanes on planet Earth
In the atmosphere there are traces of methane gas (0.0001%), produced mainly by organisms such as Archaea, which is found, for example, in the stomach of cows.
The most important commercial source for alkanes is natural gas and oil. Natural gas contains mostly methane and ethane, but also some propane and butane: oil is a mixture of liquid alkanes and other hydrocarbons. These hydrocarbons were formed when marine animals and plants (zooplankton and phytoplankton) died and sunk to the bottom of ancient seas and covered with sediment in a wikt:anoxic (oxygen-deprived) medium and covered for several million years at high temperatures. and pressure to its current form. Natural gas, for example, can be obtained from the following reaction:
- C6H12O6 → 3CH4 + 3CO2
These hydrocarbons were absorbed into porous rocks, and were located in an impermeable capsule of rock and were trapped there. Unlike methane, which is reformed in large amounts, higher alkanes (alkanes with 9 carbon atoms or more) rarely occur in large amounts in nature. These deposits, eg oil fields, have been formed over millions of years and once depleted they cannot be quickly replaced. The depletion of these hydrocarbons is the basis for what is known as the energy crisis.
Solid alkanes are known as tar and are formed when more volatile alkanes, such as gases and oil, evaporate from hydrocarbon deposits. One of the largest deposits of solid alkanes is in the asphalt lake known as Pitch Lake in Trinidad and Tobago.
Methane is also present in so-called biogas, produced by animals and decomposing matter, which is a possible renewable source of energy.
Alkanes have low solubility in water; however, at high pressures and low temperatures (such as at the bottom of the oceans), methane can co-crystallize with water to form a solid methane hydrate. Although it cannot be commercially exploited at this time, the amount of fuel energy from known methane hydrate fields exceeds the energy content of all oil and natural gas deposits combined; methane extracted from methane clathrate is then considered a candidate for future fuels.
Biological abundance
Although alkanes are present in nature in different forms, they are not biologically classified as essential materials. There are ring-sized cycloalkanes between 14 and 18 carbon atoms in musk, extracted from deer of the Moschidae family. All additional information refers to acyclic alkanes.
- Bacteria and archaea
Certain types of bacteria can metabolize alkanes: they prefer even-length carbon chains because they are easier to break down than odd-length chains. On the other hand, certain archaea, the methanogens, produce large amounts of methane as a product of the metabolism of carbon dioxide and other oxidized organic compounds. The energy is released by the oxidation of hydrogen:
- CO2 + 4H2 → CH4 + 2H2O
Methanogens are also the producers of wetland marsh gas, releasing about two billion tons of methane per year—the atmospheric content of this gas is produced almost exclusively by them. Methane production from cattle and other herbivores, which can release up to 150 liters per day, and from termites is also due to methanogens. They also produce the simpler alkanes in the human intestine. Thus, methanogenic archaea are at the extreme end of the carbon cycle, with carbon being released into the atmosphere after being fixed by photosynthesis. Our current natural gas deposits may have formed in a similar way.
- Mushrooms and plants
Alkanes also play a role, albeit a minor one, in the biology of all three groups of eukaryotic organisms: fungi, plants, and animals. Some specialized yeasts, such as Candida tropicale, Pichia sp., Rhodotorula sp., can use alkanes as a carbon or energy source. The fungus Amorphotheca resinae prefers long-chain alkanes in aviation gasoline, and can cause serious problems for aircraft in tropical regions. In plants, long-chain solid alkanes are found; they form a firm layer of wax, the cuticle, over areas of plants exposed to air. This protects the plant from water loss, while avoiding the leaching of important minerals by rain. It is also a protection against bacteria, fungi, and harmful insects—the latter sink their feet into the soft waxy substance, and have difficulty moving. The shiny coating on fruits, such as apples, is made up of long-chain alkanes. The carbon chains are generally between twenty and thirty carbon atoms in length, and are produced by plants from fatty acids. The exact composition of the wax film not only depends on the species, but also changes with the season and environmental factors such as lighting, temperature or humidity conditions.
- Animals
Alkanes are found in animal products, although they are less important than unsaturated hydrocarbons. An example is shark liver oil, which is approximately 14% pristane (2,6,10,14-tetramethylpentadecane, C19H40). Its abundance is more significant in pheromones, materials that act as chemical messengers, on which almost all communication between insects is based. In some types, such as the beetle Xylotrechus colonus, mainly pentacosane (C25H52), 3-methylpentaicosane (C26 H54) and 9-methylpentaicosane (C26H54), are transferred by bodily contact. With others, such as the tsetse fly Glossina morsitans morsitans, the pheromone contains the four alkanes 2-methylheptadecane (C18H38), 17, 21-dimethylheptatriacontane (C39H80), 15,19-dimethylheptatriacontane (C39H80) and 15,19,23-trimethylheptatriacontane (C40H82), and acts by smell over long distances, a very useful feature for pest control.
Ecological relations
An example, in which both plant and animal alkanes play a role, is the ecological relationship between the bee Andrena nigroaenea and the orchid Ophrys sphegodes; the latter depends for its pollination on the former. Bees Andrena nigroaenea use pheromones to identify a mate; in the case of A. nigroaenea, females emit a mixture of tricosane (C23H48), pentacosane (C25H52 ) and heptacosane (C27H56) in the ratio 3:3:1, and males are specifically attracted to this odor. The orchid takes advantage of this mating arrangement to cause the male bees to collect and disseminate their pollen; not only do their flowers resemble that species of bees, but they also produce large amounts of all three alkanes in the same proportion as A bees. nigroaenea female. As a result, numerous males are attracted to the flowers and attempt to mate with their imaginary mate; Although this behavior is not crowned with success for the bee, it allows the orchid to transfer its pollen, which will be dispersed with the departure of the frustrated male, to other flowers.
Production
Oil refining
The most important source of alkanes is natural gas and crude oil. The alkanes are separated in an oil refinery by fractional distillation and processed into many different products.
Fischer-Tropsch
The Fischer-Tropsch process is a method for synthesizing liquid hydrocarbons, including alkanes, from carbon monoxide and hydrogen. This method is used to produce substitutes for petroleum distillates.
Preparation in the laboratory
There is usually little need to synthesize alkanes in the laboratory, since they are often commercially available. Also due to the fact that alkanes are generally chemically and biologically unreactive, and do not undergo clean interconversions of functional groups. When alkanes are produced in the laboratory, it is usually a byproduct of a reaction. For example, the use of N-butyllithium as a base produces the conjugate acid, n-butane, as a byproduct:
- C4H9Li + H2O → C4H10 + LiOH
However, sometimes it may be desirable to convert a portion of a molecule to a functionally alkanic structure (alkyl group) using a method like the one above or similar methods. For example, an ethyl group is an alkyl group; when attached to a hydroxy group, it makes up ethanol, which is not an alkane. To convert it into an alkane, one of the best known methods is the hydrogenation of alkenes or alkynes.
- RCH=CH2 + H2 → RCH2CH3 (R = alkylo)
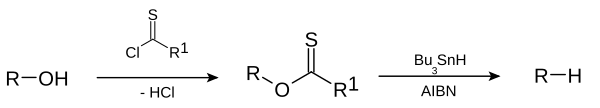
Alkanes or alkyne groups can be prepared directly from haloalkanes in the Corey-House-Posner-Whitesides reaction. The Barton-McCombie reaction removes the hydroxyl group from alcohols, for example.
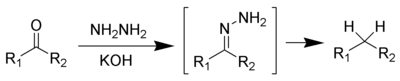
and the Clemmensen reduction or Wolff-Kishner reduction remove carbonyl groups from aldehydes and ketones to form alkanes or substituted alkyl compounds:
Other methods to obtain alkanes are the Wurtz reaction and Kolbe electrolysis.
Physical properties of alkanes
Boiling Point
Alkanes experience intermolecular van der Waals forces and when these forces are lower, the boiling point increases, and alkanes are characterized by having single bonds. See Heneicosan.
There are two determinants of the magnitude of van der Waals forces:
- The number of electrons surrounding the molecule, which increases with the molecular mass of the alcano.
- The surface area of the molecule.
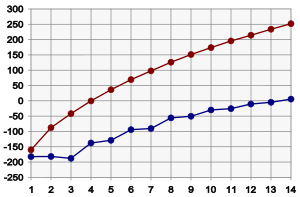
Under standard conditions, alkanes from CH4 to C4H10 are gases; from C5H12 to C17H36 are liquids; and those after C18H38 are solid. Since the boiling point of alkanes is primarily determined by weight, it should come as no surprise that boiling points have a nearly linear relationship with the molecular mass of the molecule. As a quick rule of thumb, the boiling point increases by 20-30°C for every carbon atom added to the chain; this rule applies to other homologous series.
A straight-chain alkane will have a higher boiling point than a branched-chain alkane, due to the larger contact surface area, resulting in greater van der Waals forces, between adjacent molecules. For example, compare isobutane and n-butane, which boil at -12 and 0 °C, and 2,2-dimethylbutane and 2,3-dimethylbutane, which boil at 50 and 58 °C, respectively. In the latter case, two molecules of 2,3-dimethylbutane can "fit together" mutually better than 2,2-dimethylbutane molecules to each other, so there are greater van der Waals forces.
Cycloalkanes, on the other hand, tend to have higher boiling points than their linear counterparts, due to the molecules' fixed conformations, which provide planes for intermolecular contact.[citation needed]
Melting Point
The melting point of alkanes follows a similar trend to the boiling point. That is, (all other things being equal), the larger molecule corresponds to the higher melting point. There is a significant difference between melting points and boiling points: solids have a more rigid and fixed structure than liquids. This rigid structure requires energy to be able to break during fusion. So the better built solid structures will require more energy for fusion. For alkanes, this can be seen in the graph above. Odd-length alkanes have slightly lower melting points than expected, compared to even-length alkanes. This is because even-length alkanes pack well together in the solid phase, forming a well-organized structure, which requires more energy to break down. Odd-length alkanes pack less efficiently, so disordered packing requires less energy to break.
The melting points of branched-chain alkanes can be higher or lower than that of alkenes
Conductivity
Alkanes are poor conductors of electricity and are not substantially polarized by an electric field.
Solubility in water
They do not form hydrogen bonds and are insoluble in polar solvents such as water. Since the hydrogen bonds between individual water molecules are farther apart than an alkane molecule, the coexistence of an alkane and water leads to an increase in molecular order (reduction in entropy). Since there are no significant bonds between water molecules and alkane molecules, the second law of thermodynamics suggests that this reduction in entropy would be minimized by minimizing contact between the alkane and water: alkanes are said to be hydrophobic (repel water).
Solubility in other solvents
Its solubility in nonpolar solvents is relatively good, a property called lipophilicity. For example, different alkanes are miscible with each other in all other proportions.
Density
The density of alkanes usually increases as the number of carbon atoms increases, but remains lower than that of water. Consequently, alkanes form the top layer in an alkane-water mixture.
Molecular Geometry
The molecular structure of alkanes directly affects their physical and chemical characteristics. It is derived from the electronic configuration of carbon, which has four valence electrons. The carbon atoms in alkanes are always sp3 hybridized, which means that the valence electrons are in four equivalent orbitals, derived from the combination of the 2s orbital and the 2p orbitals. These orbitals, which have identical energies, are spatially oriented in the shape of a tetrahedron, with an angle of arccos(-1/3) ≈ 109.47° between them.
Bond Lengths and Bond Angles
An alkane molecule has only C – H and C – C single bonds. The former result from the overlap of an sp3 orbital of the carbon atom with the 1s orbital of a hydrogen atom; the latter from the overlap of two sp3 orbitals on different carbon atoms. The bond length is 1.09×10−10 m for a C – H bond and 1.54×10−10 m for a C – C bond.
The spatial arrangement of the bonds is similar to that of four sp3 orbitals; they are arranged tetrahedrally, with an angle of 109.47° between them. The structural formula that represents bonds as being at right angles to each other, while common and useful, does not correspond to reality.
Conformations
The structural formula and bond angles are usually not enough to describe the geometry of a molecule. There is one degree of freedom for each carbon-carbon bond: the twist angle between the atoms or groups attached to atoms at either end of a bond. The spatial arrangement described by the angles of torsion of the molecule is known as its conformation.
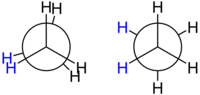
Ethane is the simplest case for studying the conformations of alkanes, since there is only one C – C bond. If you look along the C – C bond, you will have the so-called Newman projection. The hydrogen atoms on both the front carbon atom and the back carbon atom have a 120° angle to each other, resulting from the projection of the base of the tetrahedron onto a flat surface. However, the twist angle between a given hydrogen atom on the front carbon and a given hydrogen atom on the back carbon can vary freely between 0° and 360°. This is a consequence of the free rotation around the carbon-carbon bond. Despite this apparent freedom, there are only two major limiting conformations: the eclipsed conformation and the staggered conformation.
The two conformations, also known as rotamers, differ in energy: the staggered conformation is 12.6 kJ/mol lower in energy (hence, more stable) than the eclipsed conformation (less stable). The difference in energy between the two conformations, known as the torsional energy, is low compared to the thermal energy of an ethane molecule at room temperature. There is constant rotation around the C-C bond. The time taken for an ethane molecule to pass from the alternate conformation to the next, equivalent to rotating one CH3 group by 120° relative to another, is of the order of 10− 11 seconds.
The case of larger alkanes is more complex, but it is based on the same principles, with the antiperiplanar conformation being more favored around each carbon-carbon bond. For this reason, alkanes often show a zigzag arrangement on diagrams or models. The actual structure will always differ somewhat from these idealized forms, because the differences in energy between the conformations are small compared to the thermal energy of the molecules: alkane molecules do not have a fixed structural form, although models suggest so..
| Name | Formula | Pto.Ebu/orC | Pto. Fus./orC | Density g/cm -3(20.orC) |
| Methane | CH4 | -162 | -183 | gas |
| Etano | C2H6 | -89 | -172 | gas |
| Propane | C3H8 | -42. | -188 | gas |
| Butano | C4H10 | -0.5 | -135 | gas |
| Pentano | C5H12 | 36 | - 130. | 0.626 |
| Hexano | C6H14 | 69 | -95 | 0.659 |
| Heptano | C7H16 | 98 | -91 | 0.684 |
| Octan | C8H18 | 126 | -57 | 0.703 |
| Nonano | C9H20 | 151 | - 54 | 0.718 |
| Dean | C10H22 | 174 | - 30 | 0.730 |
| A dean | C11H24 | 196 | -26 | 0.740 |
| Dodean | C12H26 | 216 | -10 | 0.749 |
| Icosano | C20H42 | 343 | 37 | solid |
Spectroscopic Properties
Virtually all organic compounds contain carbon-carbon and carbon-hydrogen bonds, thus displaying some characteristics of alkanes in their spectra. Alkanes are distinguished by not having other groups and, therefore, by the "absence" of other spectroscopic features.
NMR Spectroscopy
The proton resonance of alkanes is usually found at δH = 0.5 – 1.5. The resonance of carbon-13 depends on the number of hydrogen atoms attached to the carbon: δC = 8 – 30 (primary, methyl, -CH3), 15 – 55 (secondary, methylene, -CH2-), 20 – 60 (tertiary, methine, C-H) and quaternary. The carbon-13 resonance of quaternary carbon atoms is characteristically weak, due to the lack of nuclear Overhauser effect and the long relaxation time, and may be absent in spectra from dilute samples or in which no signal has been stored for a long time. long enough.
Mass spectrometry
Alkanes have high ionization energy, and the molecular ion is generally weak. The fragmentation pattern can be difficult to interpret, but, in the case of branched-chain alkanes, the carbon chain breaks preferentially at tertiary and quaternary carbon atoms, due to the relative stability of the resulting free radicals. The fragment resulting from the loss of only one methyl group (M-15) is usually absent, and other fragments are usually spaced at intervals of fourteen mass units, corresponding to the sequential loss of CH2 groups..gg.
Chemical properties
In general, alkanes show relatively low reactivity, because their carbon bonds are relatively stable and cannot be easily broken. Unlike many other organic compounds, they do not have a functional group.
They only react very poorly with ionic or polar substances. The acidity constant for alkanes has values less than 60, consequently they are practically inert to acids and bases. Its inertia is the source of the term paraffins (meaning "lack of affinity"). In crude oil, alkanes molecules remain chemically unchanged for millions of years.
However, redox reactions of alkanes are possible, particularly with oxygen and halogens, since the carbon atoms are in a strongly reduced condition; in the case of methane, the lowest possible oxidation state for carbon (-4) is reached. The reaction with oxygen leads to smokeless combustion; with the halogens, to the substitution reaction. In addition, alkanes interact with, and bind to, certain transition metal complexes (see: carbon-hydrogen bond activation).
Free radicals, molecules with an odd number of electrons, play an important role in most alkanic reactions, such as cracking and reforming, where long-chain alkanes are converted to short-chain alkanes, and the straight chain alkanes in the branched isomers, respectively.
In highly branched alkanes, the bond angle can differ significantly from the optimal value (109.47°) to allow the different groups enough space. This causes a strain on the molecule known as steric hindrance, and can substantially increase reactivity.
Reactions with oxygen
All alkanes react with oxygen in a combustion reaction, although it becomes more difficult to ignite as the number of carbon atoms increases. The general equation for complete combustion is:
- CnH2n+2 + (1.5n+0.5)O2 → (n+1)H2O + nCO2
In the absence of sufficient oxygen, carbon monoxide or even carbon black can be formed, as shown below:
- CnH(2n+2) + 1⁄2 nO2 → (n+1)H2 + nCO
for example methane:
- CH4 + 2O2 → CO2 + 2H2O
- CH4 + O2 → CO + 2H2O
See table of heat of formation of alkanes for detailed information.
The standard enthalpy change of combustion, ΔcHo, for alkanes increases approximately at 650 kJ/mol for each CH2 group in a homologous series. Branched chain alkanes have lower ΔcHo values than straight chain alkanes of the same number of carbon atoms, so they can be seen as somewhat more stable.
Reactions with halogens
Alkanes react with halogens in the so-called radical halogenation reaction. The hydrogen atoms of the alkane are progressively replaced by halogen atoms. Free radicals are the species that participate in the reaction, which generally leads to a mixture of products. The reaction is highly exothermic, and may result in an explosion.
These reactions are an important industrial pathway for halogenated hydrocarbons.
Experiments have shown that all halogenation produces a mixture of all possible isomers, indicating that all hydrogen atoms are capable of reacting. However, the mixture produced is not a statistical mixture: the hydrogen atoms secondobromination of propane:
Cracking
Cracking breaks large molecules into smaller units. This operation can be carried out with a thermal method or a catalytic method. The thermal cracking process follows a homolytic reaction mechanism with the formation of free radicals. The catalytic cracking process involves the presence of an acid catalyst (generally solid acids such as silica-alumina and zeolites), which promote heterolysis (asymmetric cleavage) of the bonds, producing pairs of oppositely charged ions, generally a carbocation and the anion. hydride, which is very unstable.
Alkyl free radicals and carbocations are highly unstable, and undergo chain rearrangement processes, and C-C bond cleavage at the beta position, as well as intramolecular and extramolecular hydrogen or hydride transfers. In both types of processes, the intermediate reagents (radicals, ions) are permanently regenerated, so they proceed by a chain self-propagation mechanism. Eventually, the chain of reactions ends in a recombination of ions or radicals.
Isomerization and reforming
Isomerization and reforming are processes in which straight-chain alkanes are heated in the presence of a platinum catalyst. In isomerization, alkanes are converted to their branched-chain isomers. In reforming, alkanes are converted to their cyclic forms or to aromatic hydrocarbons, releasing hydrogen as a byproduct. Both processes raise the octane rating of the substance.
Other reactions
Alkanes react with steam in the presence of a nickel catalyst to produce hydrogen. Alkanes can be chlorosulfonated and nitrated, although both reactions require special conditions. The fermentation of alkanes to carboxylic acids is of technical importance. In the Reed reaction, sulfur dioxide and chlorine convert hydrocarbons to sulfonyl chlorides, in a process induced by light.
Applications
The applications of alkanes can be determined fairly well according to the number of carbon atoms. The first four alkanes are used primarily for heating and cooking purposes, and in some countries for electricity generation. Methane and ethane are the main components of natural gas; They are normally stored as gases under pressure. It is, however, easier to transport them in a liquid state, which requires compression and cooling of the gas.
Propane and butane can be liquids at moderately low pressures and are known as liquefied petroleum gases (LPG). For example, propane is used in the propane gas burner, butane in disposable cigarette lighters. These two alkanes are also used as propellants in sprays. From pentane to octane, alkanes are reasonably volatile liquids. They are used as fuels in internal combustion engines, since they can quickly vaporize upon entering the combustion chamber, without forming droplets, which would break the uniformity of combustion. Branched-chain alkanes are preferred, since they are less susceptible to premature ignition, which causes engine rattling, than their straight-chain analogues. This propensity for premature ignition is measured by the octane rating of the fuel, where 2,2,4-trimethylpentane (isooctane) has an arbitrary value of 100, and heptane has a value of zero. In addition to their use as fuels, medium alkanes are good solvents for nonpolar substances.
Alkanes from hexadecane onwards are the most important components of fuel oil and lubricating oil. The function of the latter is also to act as anti-corrosion agents, since their hydrophobic nature means that water cannot reach the surface of the metal. Many solid alkanes find use as paraffin wax, for example in candles. This should not be confused with true wax, which consists mainly of esters.
Alkanes with a chain length of about 35 or more carbon atoms are found in bitumen, which is used, for example, to pave roads. However, higher alkanes are of little value, and are usually broken into lower alkanes by cracking.
Some synthetic polymers such as polyethylene and polypropylene are alkanes with chains containing hundreds of thousands of carbon atoms. These materials are used in countless applications, and millions of tons of these materials are manufactured and used each year.
Risks
Methane is explosive when mixed with air (1 – 8% CH4) and is a very strong agent in the greenhouse effect. Other minor alkanes also form explosive mixtures with air. Light liquid alkanes are highly flammable, although this risk decreases with increasing carbon chain length. Pentane, hexane, heptane and octane are classified as dangerous for the environment and harmful. The straight chain isomer of hexane is a neurotoxin.
Contenido relacionado
Molecular biology
Bromus
Amaryllidoideae