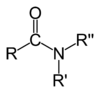
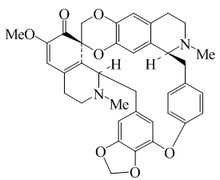
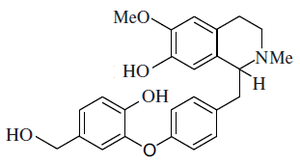
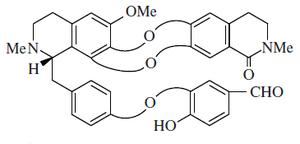
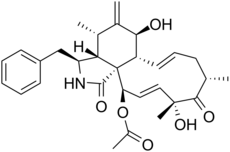
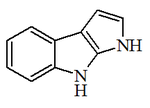
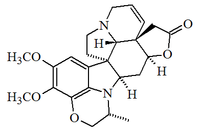
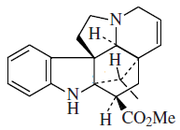
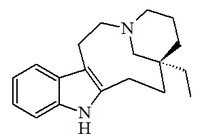
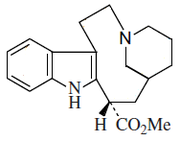
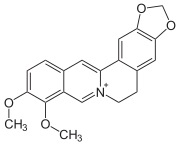
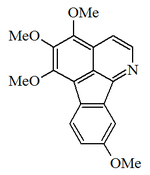
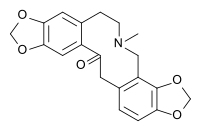
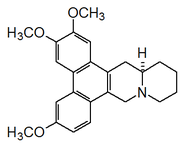
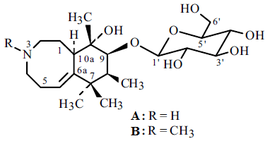
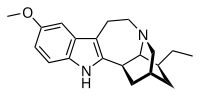
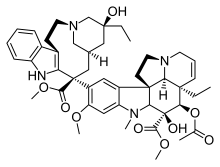
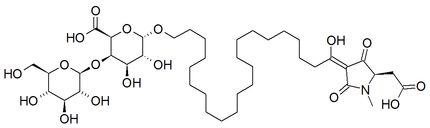
Alkaloid
It's called alkaloids (from alkali, "carbonates of alkalis", and -oid, " similar to", "in the form of") to those secondary metabolites of plants synthesized, generally, from amino acids, which have in common their water solubility at acidic pH and their solubility in organic solvents at pH alkaline. True alkaloids derive from an amino acid; therefore they are nitrogenous. All those with the amine or imine functional group are basic. Most of the alkaloids have intense physiological action in animals, even at low doses with psychoactive effects, which is why they are widely used to treat mental problems and relieve pain. Known examples are cocaine, morphine, atropine, colchicine, quinine, caffeine, strychnine and nicotine.
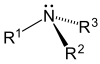
Their chemical structures are varied. An alkaloid is considered to be, by definition, a chemical compound that has a heterocyclic nitrogen from the metabolism of amino acids; if it proceeds from another route, it is defined as a pseudoalkaloid.
History and evolution of the term
The name alkaloid was coined by the German chemist Carl Meissner in 1819 to refer to natural products of plant origin that showed basic properties similar to alkalis. Given the limited structural information at the time, Meissner's definition was vague. Königs reserved the name “alkaloid” for basic compounds related to pyridine, and Guereschi considered the term synonymous with “vegetable base”. Winterstein and Trier (1910) considered alkaloids in the broad sense to be all compounds from any living being that contain basic nitrogen. These authors distinguished between a true alkaloid and a base related to alkaloids. A compound, according to this definition, had to meet the following requirements:
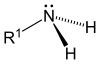
- Submit a basic nitrogen
- Nitrogen should be included in a heterocyclic system
- Having a complex structure
- Present powerful pharmacological activity
- Have a restricted distribution to plants
As studies on natural products have progressed, compounds have been discovered that are considered alkaloids, but do not meet any of these requirements: many alkaloids do not present heterocyclic systems, their nitrogen is not basic (like nitro groups), they can present simple structures (as in the case of ephedrine, many amides —such as capsaicin—), they can be pharmacologically inert, and many alkaloids have been isolated from animals.
Hegnauer (1960) classified alkaloids into three types: true alkaloids, pseudoalkaloids and protoalkaloids. The term dry alkaloid is inferred from the nomenclature of natural products and is considered here as a fourth category:
- (a) True Alkaloids: Side metabolites possessing heterocyclic nitrogen, and their carbon skeleton comes, partial or totally, from a protein amino acid.
- (b) Pseudoalcaloids: Side metabolites that possess a nitrogen, but which have not been biosynthesized from amino acids, but are formed by transfer of nitrogen in the form of ammonia to a compound of terpenic origin, steroid, polycetic, monosaccharide or a fatty acid.
- (c) Protoalcaloids: Side metabolites that do not form a heterocyclic system and are formed from a protein amino acid. Many of these compounds contain an amino, amida, etc.
- (d) Secoalcaloids: Alkaloids that come from a real alkaloid, but in which by split of the heterocyclic ring is formed an open chain nitrogenated group.
- (e) Genalcaloids: —or alkaloid aminated oxides — are derived by oxidation of alkaloids containing the R=(NO)-R group, where nitrogen has oxidation number +5, in contrast to normal alkaloids, where it is trivalent (R=N-R). His action is the same as that of the alkaloid from which they come, but it is more paused. They are named adding the gen prefix to the name of the alkaloid. Some genoalkaloids are found in nature, such as the geneserine (derivate of the alkaloid thatrine (fisostigmina)) present in the haba of Calabar.
There are natural products whose consideration as alkaloids is under debate:
- Nitrogenated bases (Adenine, thymine, guanine, uracyl and cytosine due to their primary role as nucleotides and nucleic acids.
- Them non-proteinian amino acids because their biological activity is not by joining a cellular receptor site, but because they replace protein amino acids and consequently form defective proteins.
- Sphingolipids, because they are cell membrane components.
- Vitamins (especially those of complex B) for being considered primary metabolites with catalytic activity.
- Cyanogenic glycosides because their biological activity is not by joining a cellular receptor site, but because hydrolysing produces cyanideic acid, which is the one with the biological activity.
- Glucosinolates because their biological activity is not by linking to a cellular receptor site, but because hydrolysing produces isotiocyanates, which are those that present the biological activity.
- Them aminoazúcares, such as glucosamine, for being considered rather as glucids.
- Them tetrapirrolessuch as porphyrin and corrin are still in debate, as some of them are secondary metabolites (such as turacin) and others are considered primary because metals such as iron (Hemo) magnesium (Clorophiles) or copper (Citocromines) and operate as oxygen or electron conveyors.
- Non-ribial peptides, anhydropeptides and betalactamic antibiotics remain in debate, as they may be referred to as alkaloids or not. They will be considered in this article only as structural prototypes.
The isolation of the first alkaloids in the 19th century roughly coincided with the introduction of the percolation process for the drug extraction. The French pharmacist Charles Derosne probably isolated the alkaloid later called narcotine in 1803, and the pharmacist Friedrich Sertürner investigated opium and isolated morphine. This was quickly followed by the isolation of other alkaloids such as strychnine, caffeine, among others.
Cocaine is the oldest alkaloid in terms of its structure and synthesis being established, but others, such as colchicine, took more than a century for their structures to be defined.
In the Mesoamerican area, a wide variety of alkaloids have been used in traditional Mayan medicine since ancient times. Psychotropic substances, both alkaloids and alcohols, have been used for more than two thousand years for medicinal purposes and in ceremonial rituals. Their use is regulated by women over 39 years of age (3 x 13 cycles of biological evolution according to their own scientific approach), and they are normally administered in a ceremonial way in which the person who ingests them is surrounded by other specialized members of the community.
Biological activity
Its biological activities are important due to its hormonal mimicry and its intervention in the main reactions of cell metabolism. Despite being substances that are not very similar to each other from a structural point of view, they have similar physiological properties. Many alkaloids are the cause of poisoning in humans and animals. The most common form is poisoning from herbal infusions for medicinal purposes, this being an important cause of death, especially in children. Its presence in vegetables makes it possible to accidentally incorporate it into food, creating an easy way of poisoning.
They generally act on the central nervous system, although some affect the parasympathetic nervous system and others the sympathetic nervous system, for example, cocaine acts by preventing the reuptake of dopamine from the synaptic terminal, which produces a greater effect of the dopamine receptors.
The biological activity of alkaloids is very diverse; the most studied is the euphoric action that some have, such as cocaine, although there are also alkaloids with depressant effects on the central nervous system such as morphine.
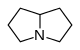
Pyrrolidine alkaloids are associated with pyrrolizidine structures; they are secondary metabolites of a wide variety of plants, including species found throughout the world. These plants are the cause of numerous cases of cattle poisoning, and have caused great economic losses. They are also a cause of death in humans, especially in underdeveloped countries, as a consequence of the contamination of cereals and seeds, which is why they are of great importance in the field of food. It is believed that the ingestion of herbs and vegetables that contain these alkaloids cause ailments. The structure of these alkaloids consists of two rings of 5 linked atoms that share a nitrogen atom. In nature, rings generally have hydroxymethylene groups in the c-1 position and hydroxyl groups in c-7 as substituents; this structure is known as a necina. Typical examples of this base are heliotridine and retronecin.
Phytochemistry
Extraction methods are very varied, but purification by means of supercritical fluids, specifically with carbon dioxide, has recently been gaining momentum. To obtain plant alkaloids, they are extracted from the parts of the plant that contain them, with water if they are in the form of salts (soluble) or with dilute hydrochloric acid if they are in the insoluble form.
Regarding their detection, there are many methods: chromatographic procedures, colored reactions (Mayer, Dragendorff, Bouchardat reactions... although they are not specific to alkaloids: a positive result can be obtained in the presence, for example, peptides). Spectroscopic methods such as mass spectrometry, nuclear magnetic resonance and infrared spectroscopy are currently used.
Classification by chemical structure
Non-heterocyclic compounds
Since non-heterocyclic pseudoalkaloids, protoalkaloids, and secoalkaloids do not have a heterocyclic structure, they can be considered to have a highly diverse hydrocarbon structure. In this section these compounds will be classified by their functional group:
| Functional group | Structure | Examples |
|---|---|---|
| Primary Amine | biogenic amines, anthranic acid, p-aminobenzoic acid, bleniona. | |
| Secondary Amine (R1 = H) or tertiary | Spermine, spermidine, sarcosine, peshawarina, narcein, joubertiamine, cordiformmide, lilacinona, lepiotaquinone, tetracyclines, taspina. | |
| Sal de amon cuaternaria | Betains, muscarins. | |
| Primary | Anandamida, nicotinamide, cerulenin, agro-cook. | |
| Secondary and tertiary | Non ribosome peptides. Pigs.,,,,................................. | |
| Guanidina | Galegine, creatine, hyrudonine, spherodies, fontainein | |
| Nitrilo | cyanogenic glycosides | |
| Dip | Kinamicins, agaritine, gyramitrine, headingflavina, craniformin, stephanosporine. | |
| Azoxi | Cycsine, azoxybacillin | |
| Nitro | β-nitropropyonic acid and its esters, cloranphenyl, aristocycic acid, 1-amino-2-nitrocyclopentanocarboxylic acid, 3-nitro-4-hydroxyphenylacetic acid, aureotin, myerotoxin, ascoclavine | |
| Hydroxylamine and oxyma | Deferrioxamines B, deferoxamines, hadacidina, lobatamides |
Heterocyclic Structures
Alkaloids and pseudoalkaloids will be classified below according to their heterocyclic structures.
| Name | Skeleton | Examples |
|---|---|---|
| Aziridina | Acid 2,3-aziridindicarboxylic, disidazirin. | |
| Azetidina | Azetidin-2-carboxylic acid, mugineic acid, 3-azetidinone, 3,3-azetidinodiol, SQ 26,180, nicotianamina, penaresidines, penazetidines, calidafninone, betalactamic antibiotics. | |
| Pirrol | Pirrolidins: Cuzcohigrina, higrina, higrolina, stachidrina, ficina, vochisina, eleocarpine, dendrocrisanins, agaricone, pirrocyclines, chabamida F, equisetin, fomasetin, salinosporamides, pseurotins, kafiinic acid, ruspolinona, gerrardina, aminopirrolodin1 and C2, anisomycin, antibiotic GKK 1032A2, barmumicin, brousonetins, codonopsine, anisomycin, cucubalactama, detoxin, hydroxypirrolidins, divaricataester A, donaxaridine; dysysybetin, fulvanine A, involved, irin, lanopilins, morphine
Pyrroles, Pyrrolonas and Succinimides: Pyrolocarboxylic acid, tenuazine, porphyllins, porphylline, prodigyins, ryanodine, pirrolezantine, 3-pirrolacrylic acid, 2-cyanopirrol, chistamidins, pharynomycin, pirrolo-2,3-dicarboxylic acid | |
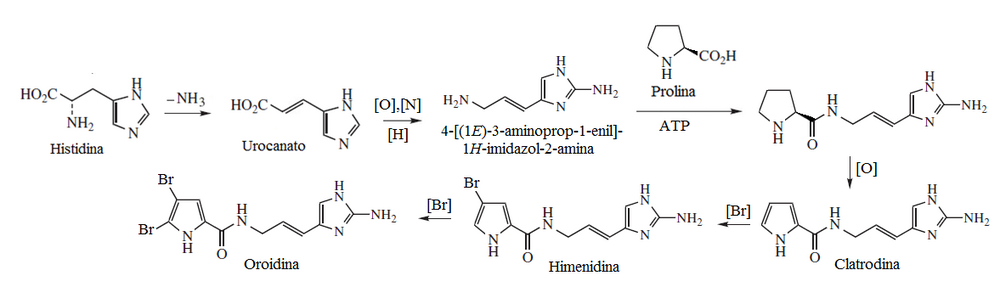
| Imidazol | Urinanic acid, histamine, murexine, diphetamine, ergotionine, pilocarpine, dolicoteline, pilosin, nagelamides, sceptrines, clatrodine, oroidine, himenidine, keramadina, creatinine, polycarpene, steletazoles, chrysorinas, lepidines. | |
| Pirazol | β-pirazole-1-il alanine, pirazophrine | |
| Oxazol | Oxazolidins: Goitrina, lipoxazolidinonas
Oxazoles: Muscazona, hennoxazoles, rhizoxin, forboxazole, anulolina, halfordinol, texalin, texamine, vibriobactin, curromycin A, martephramines, Almazolone, clavosins, quivosazoles. | |
| Isoxazole | Isoxazolidins: Cycloserin
Isoxazoles: Ibothenic acid, trichomal acid, thighs. | |
| Tiazol | Epotilonas, thiamine, micrococcina, mixotiazoles, nostociclamides, thiostreptona, raocilamides, dendroamides, tenuacilamides, yersiniabactin, healers, venturamides. | |
| 1.2.4-oxadiazol | Qualic acid | |
| Piridina | Piperidinas: Coniine, conicein, pinidine, lobelanine, pipecolic acid, glutanimin, indigoidine, sedamine, lobelin, anaferin, piperin, nufaramine, carpain, estenusin, evoninic acid, wilphineic acid, evonine, rohitukin
Piridines:............. | |
| Pirimidina | Ectoin, capreomicidine, thymine, cytosine, uracyl, vicin, convicin, latirin, barbiturate acid | |
| Pirazina | Pulcherrymic acid, asperegylic acid, ligustrazine, riterazins, anhydropeptides, chromtonin, dredmacidins, sarcodonins, sarcoviolins, abornousin, picrorocceline, rhodotoric acid, echinine, flavacol, micelianamida, barrenazins. | |
| Piridazina | Piridazomycin, Piridazocidina | |
| Tiomorfolina | Condrine, thiomorphine-3-carboxylic acid, cycloaliine | |
| Azepina | Azepanos'Blessings, isobengamides, muscaflavina, Ciliatamides, ofiocordine, balanol, chalciporone, peritoxin A, ustalimida, amamistatin A, antibiotic A 500359A, balanol, calpinactama, capuramicin, carboxymicbactins, ciliatamide A, circintocin | |
| Azocina | Otonecina | |
| Azacicloalcans | Motuporaminas, macrocyclolactamas, Keramafidina C | |
| 7-azabicycle[2.2.1]heptano | Epibatidina | |
| Tropano | Atropine, scopolamine, hyosciamine, cocaine, ecgonine, calistegines | |
| 9-Azabiciclo[3.3.1]nonano | Pseudopeletierin, eufococcinin | |
| Pirrolizidine | Necinas: Retronecina, heliotridina, laburnine, indicine, lindelofina, sarracina, platifilina, tricodesmina, phenopsine, licopsamina, creatonotins, calimorfina, supidina, rosmarinecin, danaiona, traquelantamidine, niece, crotanecina, platinecin, turneforcidina, | |
| Indolizidine | Swainsonine, castanospermine, pumiliotoxins, lentiginosine, polygonatins, monomorine, slaframine, serratinine. | |
| Indol | Methyl, methyl, methyl | |
| Isoindol | Cytocalasins, quetoglobosins, cespitulactamas, hericerin, esrenins, cytocytes. | |
| Benzotiazol | Luciferina | |
| Benzoxazol | Nakijinol, pseudopteroxazoles | |
| Indazol | Nigelidina, nigelicine, nigeglanin | |
| Pirrolo[3,2-b]piridina | Laccarina, agrocibenin, pyramids | |
| Pirrolo[1,2-a]pirazine | Vercapamida A | |
| Imidazo[1,2-a]pirazine | Coelenteracina, vargulina | |
| Quinolina | 'Hydroquinolins: Mirionine, tortuosamine
'Quinolins: 2nd, 4th, 4th, 2nd, | |
| Isoquinolina | Tetrahydroisoquinolinskinabine, phylocriptine, filthine,
Isoquinolinas: Isoquinolin, salsoline, lofocerine, coridaldine, oxihydrstinine, ancistrocladines, papaverin, laudanosine, sendaverine, papaveraldine, carcrisine B, fusarimida, cassiarinas, monascorubramine, fredericamicins A and B, lagoonmicin. | |
| Quinolizidina | Lupinine, nufaridine, nufarolutin, nufacristine, nufarpumilamins. | |
| quinazolina | Febrifuguine, glycoin, arborine, glycosminine, glycoscimine, glycophymoline, glomerin, homoglomerin, equinozolinone, tetrodotoxin, 2-acetilquinazolin-4(3H)-one, 7-bromoquinazolinoline-2,4-diona, 7-hydroxyequinozolinone, ditioquinazoline. | |
| Quinoxine | Baimantuoluoamida B | |
| 3H-Pirrolo[1,2-a]azepine | Estemoadina | |
| 3H-3-benzazepina | Roeadina, papaverubinas | |
| 2,7-Naftiridina | Lofocladinas | |
| Purina | Xantosina, adenine, guanina, cordicepine, eritadenins, nebularin, cytokinins, caffeine, teofiline, teobromine. | |
| Pteridine | Biopterins, leucopterina, xantopterina | |
| Pirimido[5,4-e][1,2,4]triazina | Reumitsina, toxoflavina. | |
| Pirazolo[4,3-e][1,2,4]triazina | Fluvial | |
| Carbazol | 3-Methylcarbazole, 3-Formilcarbazole, 3-carbazolecarboxylic acid, 1-Hidroxi-3-methylcarbazole, clausins, murrayafolins, koenolina, murrayanine, mukoenic acid, mukonine, 2-hidroxi-3-methylcarbazole, mukonal, mukonidine | |
| Acridina | Rutacridona, melicopidina, Xantevodina, eskimianina. | |
| Fenazina | Piocianine, aeruginos. | |
| Fenoxazina | Cinabarin, cinnabarnic acid, tramesanguina, polystictin, phenoxazon, α-aminophenoxazone, orceins, picnoporin. | |
| Benzog]isoquinolina | Tolipocladine | |
| Benzog]quinolina | Cleistofolina | |
| 4H-1,4-benzoxazina | DIMBOA | |
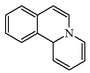
| Quinuclidina | Quinine quinidina fivenine, cinchonidine. | |
| 1,2-Dihydrospiro[indol-3,3'-pirrolidina] | Coerulescina, Horsfilina | |
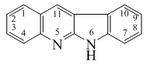
| β-Carbolina | Harmano, harmina, harmalina, eleagnina, cecilin, tripargimina, bruneins, flazina, flazinamida. | |
| Benzo[g]pteridine | Lumazina, Limicromo, isoaloxazines, roseoflavina | |
| 7H- dibenzod.f]azonina | Protostefanine | |
| Benzoh]isoquinolina | Chiloenina, santiagonamina | |
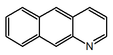
| Fenantridina | Crinina | |
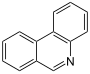
| Benzoc]cinolina | Necatorine | |
| Pirido[1,2-a]indol | Gliotoxin, dioxopirazinoindoles | |
| Pirido[2,1,6-of"quinolizine" | Coccineline, mirrine, precoccineline, hypodamine, convergine, poranterin, porantelidina, porantericine, propilein | |
| 11bH- pissed[2,1-a]isoquinolina | Emetina | |
| Pirrolo [2,1-a]isoquinolina | Lamelarinas | |
| Pirrolo[4,3,2-of]quinolina | Micearubinas, makaluvaminas, damirona C, hematopodinas. | |
| 2H-1,8,8b-Triazaacenaftileno | Cylinderspermopsin and its derivatives. | |
| 5H-5,6,8b-Triazaacenaftileno | Ptilomicalins, batzeladins, cambrescidins. | |
| 9aH-5,8-diazabenzo[3]cd]Azuuleno | Aaptosamine | |
| 4H-benzoof][1,6]-naftiridina | Aaptamines, isoaaptamines | |
| Pirido[2,1-j]quinolina | Cylindricin B, fasicularin, polycytorol A | |
| 1H-pirrolo[2,1-j]quinolina | Cylindricins A, K, D, E, J, I; Lepadiformins A and B. | |
| 1.8-dihydropirrole[2,3-b]indol | Fisostigmina (eserin), etheramine, fisovenine, eptastigmine, flustramines. | |
| 5H10H- Spread it[1,2-a:1',2'-d]pirazine | Vercapamida C, pyrocol, aranotin. | |
| 2-oxa-6-azatriciclo[4.2.1.03.7"Nonano." | Lolinas, temulina | |
| Dibenzo[c,g]azecina | Protoxin, coricavamine, coricavidina. | |
| Dibenzo[b,i]quinolizina | Cohirsina, shaheenina, cohirsinine, cohirsitinine. | |
| Pirrolo 2,1,5-cdIndolizine | Mirmicarinas | |
| 1H10H- [1.2-c]purina | Saxitoxins | |
| Indolo[2,1-a]isoquinolina | Criptowolidina, criptowolinol, criptowolina. | |
| 9H-Indeno[2,1-b]piridina | Haouamina | |
| Azepino[3,2,1-hi.]indol | Estenine, tuberostemonine, estemoamide, estemonine, neostemonine, chromomine, estemonidine. | |
| 6,10-Methane-4,6-dihydropyride[1,2-a]azepine | Securiniaminas, sufruticodina, securinoles, filocrisine | |
| 2.5-Methane-9H-pyride[1,2-b][1,2]oxazepine | Filantidina, secuamamide D | |
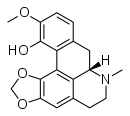
| 4H- dibenzode,g]quinolina | Alkaloids aporfinoids: Glaucina, boldina, bulbocapnine, nantein, hernandialin, nuciferin, liriodenine, pukatein, laurotetamine, lauroscolzine, scoline, magnoflorin, duguenain and pancoridine, talifaberine, urabain, dehatrifin | |
| 7H- dibenzoof,h]quinolina | Menisporfina | |
| Indeno[1,2,3-ij]isoquinolina | Rufescina, imelutein | |
| 1.2-Epimino-3H-pirrolo[1,2-a]indol | Mitomicines | |
| Eupolauridina | Eupolauridina and its derivatives | |
| Pirrolo[3,2,1-of]fenantridina | Licorina | |
| 7H-Nafto[1,2,3-ij][2,7]naftiridina | Sampangina, eupomatidines | |
| Spiro[2,5-cyclohexadieno-1,7'(1'H)-cyclopenta[ij]isoquinolin] | Orientalinone, glaziovina, estefarina, mecambrina, pronuciferin.
Number: Roehibridina | |
| 1.2-dihydrospiro[2-H-indeno-2,1'-isoquinolin] | Lahorina | |
| Benzod]-[4,5-g[4,3,2-jk][2]benzazepina | Dragabina | |
| Esparte | Esparte | |
| 1H.4H.9H-Dipirted[2,1-b:3',2',1'-ij]quinazolina | Sibiridina, schoberina | |
| Morphine | Codeine, tebaine, salutine, morphine, oripavina | |
| Hasubanano | Hasubanonine, cepharamine, cephasamine | |
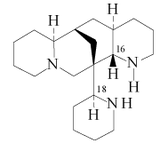
| (1S.9R)-7,11-diazatriciclo[7.3.1.02.7]tridecano | Citisine, angustifolina | |
| Dibenzo[f,h]pirrolo[1,2-b]isoquinolina | Ficuseptins, cryptopleurine and thiophorin. | |
| (6S,11bS)-6,11b-Methane-3a,6,11a,11b-tetrahidrofuro[2,3-c[1,2-a#Balepine | Securiniaminas, sufruticodina and A-D securinoles | |
| Dibenzoa,g]-9aH- What? | Protoberine, anisocycline, palmatin, corlipalmine, discretamine, berlambina, lambertine, choreximine, talifaurine, coptisine, escoulerin, styling. | |
| 6,15-epimino-4H-Ioquino[3,2-b][3]Benzazocina. | Saframicina, renieramicina, jorunamicina | |
| (1R,2R,7S,12R,13S,15S)-14-Oxa-6,8-diazapentaciclo[10.6.0.02.7.02,15.08.13Octadean
(Nitraramida) | Nitraramida, nitrabirina | |
| 3.5-o-fenilen-2,3,4,5-tetrahydro-1H-2-benzazepina | Amurensina | |
| 3.6-Dihydropirrolo[3,2-e]indol | Duocarmicins, yatakemicin. | |
| 5,11-Epiminodibenzoa,e][8]anuleno | Pavinas, isopavinas | |
| 10H-blue[1,2,3-ij]isoquinolin | Imerubrina | |
| 5H-Indeno[1,2-b]piridina | Oniquina | |
| Dibenzo[6,5,4-cd:f]indol | Cefaronas | |
| 5H-Isoindolo[1,2-b][3]benzazepina | Lennoxamine | |
| Benzo[6,7]ciclohept[1,2,3-ij]isoquinolin | Kreysigina, | |
| 9H-azulene[1,2,3-ij]isoquinolina | Grandirubrina | |
| Homoeritrinano ([4,5-h]indolo[7a,1-a][2]benzazepina) | Schelhameridin, erimelantine, erisopinoforin. | |
| Eritrean | β-Erytroidine, Erisotramidina. | |
| 3H-cyclopenta[b[1.2-a][3]benzazepina | Cefalotaxine, haringtonin, isoharringtonin, cephalezomines | |
| Dibenzo[5,6-a:4',5'-g]-4H- What? | Cavidina, Talictrifolina, apocavidina, isoapocavidina. | |
| Quinolino[2',3':3,4]b]quinazolina | Luotonins | |
| Benzo[c]fenantridina | Quelidonina, sanguinarina, palmatina, queleritrina | |
| Benzo[j,k]acridina | Necatarona | |
| Indolo[3,2-c]quinolina | Isocriptolepine | |
| Quindolidina | Quindolidina, Criptolepina | |
| Quinindolina | Neocriptolepine | |
| 7H-Indolo[2',3':3,4]pyrido[2,1-b]quinazolina | Evodiamine | |
| 5H-indolo[2,3-a]pirrolo[3,4-c]carbazol | Arciriaflavinas, estaurosporine | |
| 9H-Quino[4,3,2-of][1,10]fenantrolin | Ascididemine | |
| 11,22-Diazatetracycle[11.11.2.12.22.02.12]heptacosane | Ircinate |
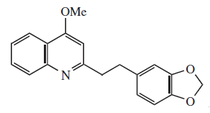
- Pyridal core alkaloids: to this group belong nicotine, pilocarpine and spatein. Nicotine is found in tobacco juice with other alkaloids. It is a colourless, smelly liquid similar to tobacco and hot and spicy flavor. It's very toxic in high doses.
- Isoquinoleic core alkaloids: they are found in papal and ranunculaceae plants. The most important is papaverin, which has hypnotic properties (although not as accentuated as morphine).
- phenantrenic core alkaloids: the most important is morphine. It is found in the opium in the form of salt. It is used in medicine in the form of chloride and sulfate, such as sedatives, analgesics, anesthetics and soothing.
- Alkaloids of tropical core: Atropine and cocaine belong to this group. The atropine is found in the juice of several plants such as the beautifuldone, brugmansia, floripondium, toloache and the estramony. Coca is extracted from coca leaves or Erytroxylium Coca, is of bitter taste, insensitizes the tongue, and is used in chloride-like medicine as a local anesthetic to cure pain and severe epistaxis.
- Alcoholic core alkaloids: the most important are strychnine and brucina. Stricnine is one of the most energetic and toxic alkaloids, extracted from various plants of the genus Strychnosamong them the haba of St. Ignatius and the Vomica nut or Nux. It is of very intense bitter taste and is an extremely toxic substance. its ingestion produces tetanical seizures.
- Undefined core alkaloids: are all those alkaloids whose constitution has not yet been clearly established. Among them is aconitin (very violent poison, used in therapeutics to combat certain ailments) and ergotinin (one of the active principles of the rye cornezuelo, which exerts a specific action on the uterus).
Classification by biosynthesis
Alkaloids are found forming salts with acetic, oxalic, lactic, malic, tartaric, and citric acids. Below is a summary of the biosynthetic diversity of alkaloids.
Protoalkaloids
Many compounds considered pseudoalkaloids could be included in the category of protoalkaloids if all those that do not form heterocyclic systems are considered. The most important ones to consider are amines and amides.
Amines
When an amino acid is decarboxylated, biogenic amines are formed. Histidine and tryptophan, as they possess a heterocyclic ring by themselves, will be considered in separate sections.
| Amino acid precursor | Amina biogena | Structure |
| Glicina | Metilamina | |
| Alanina | Etilamina | |
| Serina | Etanolamine | |
| Cistein | Cisteamine | |
| Asparian acid | β-Alanine | |
| Metionina | 4-Methylsulfuro-1-propanoamine | |
| Treonina | 1-Amino-2-propanol | |
| Glutamic acid | γ-aminobutyric acid (GABA) | |
| Ornitina | Putrescina | |
| Arginine | Agmatina | |
| Lisina | Cadaverina | |
| Fenilalanina | Fenetilamina | |
| Tyrosine | Tiramina | |
| DOPA | Dopamine | |
| Valina | Isobutilamina | |
| Leucina | Isoamilamine |
Many of these amines form further derivatives, such as catecholamines. Ephedrines are formed from benzoic acid and pyruvate by the action of thiamine pyrophosphate.
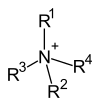
Many amines can accept a second or even a third alkyl group to form secondary or tertiary amines. When an amino acid forms a trimethylammonium salt it is called betaine. Examples of betaines are glycinic betaine, trimethylserine (the precursor to choline) and hypaphorin. Muscarine is an ammonium salt of Amanita muscaria.
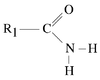
Amides
Amides are formed by the heteroatomic connection of an amine or ammonia with an acyl-coenzyme A.
Guanidines
There are amines that receive a carbimino group from arginine, such as galegine and creatine.
True alkaloids
They always have a nitrogen that is part of a heterocycle, they are basic in nature, they are normally in the salt state and biogenetically they come from amino acids.
Alkaloids derived from serine, cysteine and glycine
Serine
- La serin forms the cycloserin, an isoxazolidin-5-one by intramolecular oxidation of nitrogen.
- Seine can form acid 2,3-diaminopropyonic (acid)DAP), which in turn can form the quisqualic acid, a non-proteinnic amino acid with a ring of 1,2,4-oxadiazolidine, isolated from the piscuala. DAP also forms β-lactamas, which are constituents of SP 26 antibiotics.
- Seine can be incorporated as etanolamine to alkaloid epilacneno azamacrólide.
- In several non ribosomal peptides, seine can form oxazoles and these can be referred as alkaloid peptides. Oxazoles are the result of iclation and oxidation of non ribosome peptides of seine or throonine
Cysteine
Cysteine can form various thiazoles:
- An example is acid 2-methyl-1,3-tiazol-4-carboxylic. This thiacolic acid is the initiation unit of polycytes such as epotylins.
| Epotilone C |
- Another example of cysteine tiazoles are the Mixotiazoles, which were isolated by Höfle and his collaborators in the 1970s. Mixotiazole A was first described in 1978 in a patent and was later described in the 80's. Mixotiazoles are 2.4'-bi-1,3-tiazoles isolated from fungi that form from a polycyte with a cysteine initiation unit and another cysteine molecule.
- Cysteine can also form thiomorphins in marine organisms, such as condrine.
Wisteria
- Glicin can form imidazole rings, such as AIR and creatinine.
- From the glycine the guanine and adenine purin bases are formed. From the adenine, several purinic compounds are formed, such as cordicepine, eritadenins, nebularin, cytokinins, caffeine, teofiline, teobromine, malonganenonas and nutingins.
- From the guanine you can sistetize isoaloxazine and pteridine rings. Many insects can produce pigments from pteridins (e.g. Drosophin, Leucopterin, Drosopterina) or other derivative rings:
- La reumitsina and the toxoflavina are compounds that present as the base skeleton the pirimido[5,4-e]-1,2,4-triazine (also referred as Azapteridines). These compounds were isolated from gender bacteria Actinobacter. These triazines may come biogenetically from a purine or pteridine.
- Examples of azaguanidines is zarzisine.
| Pirimido[5,4-e][1,2,4]triazina |
Alkaloids derived from aspartate
In this section we consider the alkaloids of amino acids biosynthetically related to aspartic acid: aspartate, asparagine, threonine and methionine.
Aspartate and asparagine
Aspartate can form another related amino acid by reduction of the carboxyl terminal and reductive amination: 2,4-diaminobutyric acid (DABA). When decarboxylated, it can form 1,3-diaminopropane. Aspartate can accept a carbamyl group via its amino nitrogen to form N-carbamoylaspartic acid. Additionally, aspartic acid can condense with dihydroxyacetone phosphate to form the vitamin nicotinic acid.
- Pirimidins: The precursor is the orotic acid, which is formed by heterocyclization of the acid N- Carbamoilaspártico. Pyridic bases (Uracilo, Timina, Citosina) arise from this precursor. Barbiturate acid is the product of catabolism of pirimidins. Examples of alkaloids from orotic acid are the vicin, convicin, of Vicia faba and the cytokine Pectinophora gossypiella. Latirine could be considered as a non-protein amino acid or alkaloid due to the pirimidine ring. ectoine is a 2-replaced pirimidine that does not come from the path of orthotic acid, but from acid 2.4-diaminobutyric acetylated in carbon nitrogen 4.
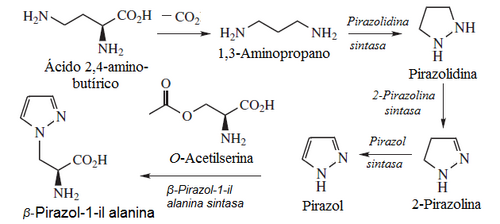
- Pirazoles: Pirazol rings are not abundant in nature, and have chemotaxonomic value. One case is β-pirazole-1-il alanine and pyrazophrine.
- Pyrid alkaloids[1,2-a]azepinic: Form in the sponge Niphates digitalis from subtheric aldehyde and 1.3-propanoamine (which comes from the descarboxylation of 2.4-diaminobutyric acid)
Threonine
Several non-protein amino acids with toxic activity—such as hypoglycins, canalin, and canavalin—are thought to be derived from threonine.
Methionine
The nitrogenous secondary metabolites of methionine mainly consist of methionine glucosinolates, homomethionine and dihomomethionine. Goitrin is a 1,3-oxazolidine formed from the glucosinolate progoitrin. S-Adenosyl methionine is reactive enough to form an azetidine system, in the form of azetidine-2-carboxylic acid, which is the basis of the isopeptides known as mugineic acids. Some azetidines are presumed to be derived from this precursor, such as 3-azetidinone and 3,3-azetidynediol.
| Acid (S)-(-)-2-Azetidinocarboxylic | Goitrina |
Nicotinate
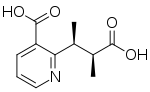
Nicotinic acid is formed de novo in plants by condensation of a triose molecule and an iminoaspartate molecule (the imino derivative of aspartic acid), while in animals and fungi it can be formed by tryptophan catabolism. Nicotinic acid (pyridine-3-carboxylic acid) is the structural basis of many pyridine alkaloids. Fusaric acid is a pyridine alkaloid that is formed under the same condensation principle, but from aspartate and triacetic acid.
- Pyridines: This vitamin can be discarded to give piridine, hydrogenated to form dihydropyridins or oxidize. Some pyridines related to nicotynic acid are the alkaloids of the palm Areca (Arecoline, guvacin), the seed of the ricin, the hermidine of the Mercurialis annua and the alholva's trinelin. Several plants of the Celastraceae family produce evoninic acid esters (nicotinic acid linked with a 2-methylbutyric acid molecule) and agarofuran type sesquiterpenos. Examples of these alkaloids are maytoline, mayine, acantotamine, evonin, neoevonin, euonimin, hypocratins, emarginatins.
| Evonic acid | General structure of the alkaloids of Celastraceae. |
- Bispiridines: The bis-pyridines are formed by the coupling of free radicals (Crisohermidina) or by condensation (Anatabine). Piridine rings can be coupled with other rings like in the case of nicotine (Pyrrolidynic ring) or anabasine (Pypiridine ring of lysine)
 | |
| Crisohermidina | Anatabine |
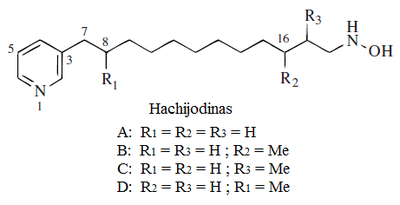
- Hachijodinas: They are hydroxylamins derived from fatty acids that form when nicotynic acid is used as an initiation unit:
Alkaloids derived from amino acids of the glutamate family
Several alkaloids come from amino acids of the glutamate family (glutamine, glutamate, proline, ornithine, and arginine.
Glutamate and glutamine
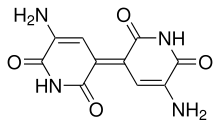
- When glutamine heterocyclizes at its ends, form the glutanimineWhich has a piperidine skeleton. When dimeriza forms the indigoidine (Estructura: 1.1',2,2',3,3',4,4'-octahidro-4,4'-bipiridina).
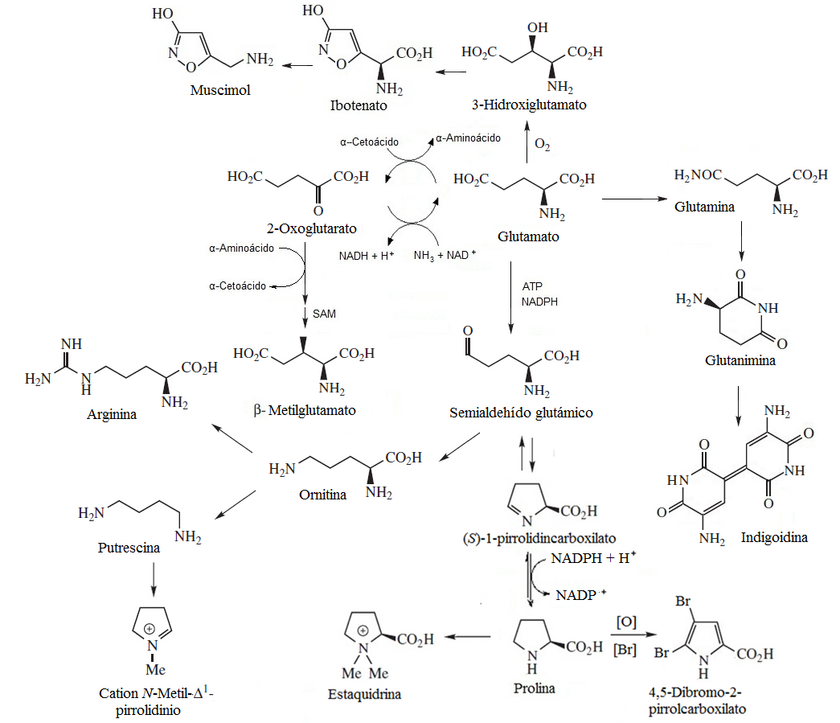
- Hydroxylate glutamate can form an isoxazole in a manner similar to cycloserin to form ibothenic acid, a non-proteinnic amino acid with an isoxazole heterocycle. This amino acid is the precursor of many mushroom isoxazoles Amanita muscaria, such as thigh and trichomal acid.
- The lascivol is a glutamine amide and a polycetic precursor of the many indian structure compounds, such as 2.4-dimetilindol, (2-methyl-4-hydroxymethylndol, (2-methyl-4-methoximetilindol and 2.4-dimethyl-5-metoxindol
Proline
Proline and ornithine can form alkaloids with a pyrrolizidine nucleus; in fact, proline itself is a pyrrolizidine. From proline can be formed:
- 2-pirrolocarboxylic acids as well as their brominated derivatives, isolated from sponges.
- Stachyrin, present in betanic, is proline betaine.
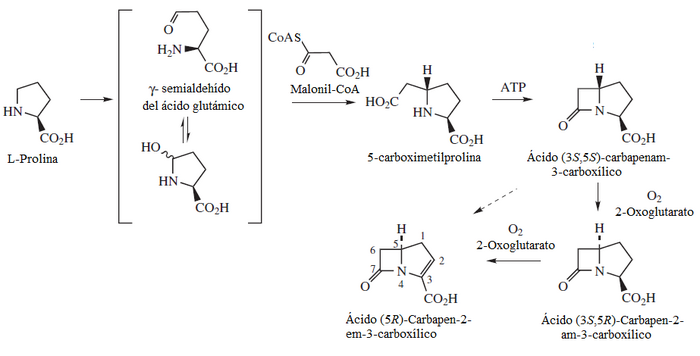
- Antibiotics beta-lactamic carbapenam type.
- Many clavicipitous fungi produce alkaloids with 2-oxa-6-azatricicle skeleton[4.2.1.03.7]nonano, such as lolina and temulin, from proline and homoserine.
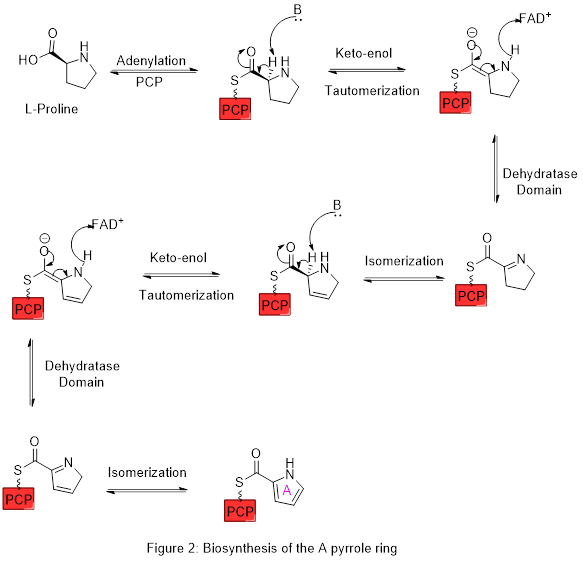
- Prodigiosins are manirlic pigments produced by gender bacteria Serratia. The first pirrol ring is biosynthesized by the dehydrogenation of a proline thyoester with the enzyme:
Subsequently, the 2-pyrrolecarbonyl residue is condensed with a new pyrrole ring of polyketide type origin:
Ornithine and arginine
Ornithine and arginine are related biosynthetically, since arginine comes from ornithine by adding a carbamyl group to the terminal nitrogen of ornithine with subsequent addition of an aspartate nitrogen (first 3 reactions of the urea cycle)
Ornithine forms alkaloids of two types: those derived from putrescine and those derived from the polyamines spermine, homospermine, and spermidine.
- Derivatives of the rot: Putrescina can heterociclize to form the cation N- methyl-Δ1- Pyrroliny.. This metabolic intermediary can be incorporated into many routes such as:
- (a) Training of 3-pirrolidin-2-il piridines, such as nicotine
- (b) Training of azaazulens, such as (+)-5-epiindolizidine 167B.
- (c) Formation of two acetyl units: By the same mechanism of the polycytes (Only the initiation unit is a magnet, so instead of a Claisen condensation a Mannich condensation is carried out. Thus form the higrine, cuscohigrine and dendrocrisanins. When the polycyte-type derivative heterocyclizes the tropane skeleton. Many tropical alkaloids form in solanaceae and erytroxylaceas, such as D., Atropa,Mandragora and Erythroxylon. Examples of these alkaloids are cocaine, hyosciamine, tropine, calistegins, schizantines, litorina, methelloidine and scopolamine.
- (d) Alkaloids pirrolidinflavonoids: The rings of N- methyl-Δ1-pirrolinium can be incorporated into flavonoid structures. Examples of these alkaloids are ficina, vochisina and eleocarpine
| Ficina | Vochisina |
- Dendrocrisanins are pirrolidine rings with substitute cinamoyl in nitrogen.
- Agaricone is a pigment produced by Agaricus xanthoderma.
- -Polyamine derivatives: The homoesperidine can give a double cycling to produce biosynthetically pirrozilidinic alkaloids. The plants of the genus Seinethe monarch butterfly (Danaus plexippus and other related lepidopteros (which consume the plant) and several orchids are the main producers of pirrolizidinic alkaloids:
Examples of these alkaloids are falenopsin, lycopsamine, retronecin, creatonotins, calimorphine, supidine, rosmarinecin, otonecin, danaione, trachelantamidine, plataphyllin, sarracin, hastanecin, crotanecin, heliotridine, platynecin, turneforcidin, ipangulines, and mynalobins.
- Many macrocyclic alkaloids come from spermine, as in the case of the lunarine (Insulation of the Lunaria annua(c):
- The structure of motuporamins suggests an origin from polyamins and a medium-sized fatty acid:
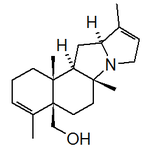
- Alkaloids of the Stemona: The species of the family Stemonaceae produce a large class of diverse alkaloids structurally related to the 4-Azaazulene core. Its roots have been used for the treatment of tuberculosis, bronchitis and parasitosis. Examples of these alkaloids are stenine, tuberostemonine, stemoamide, estemonine, neostemonine, chromomine and estemonidine.
- Alkaloids Elaeocarpus: The species of the genus Elaeocarpus produces a series of indolizidal alkaloids, but unlike those who are swansonin type, these alkaloids are presumed to come from the spermidine. They are classified into:
- - Alkaloids 8-acilindolizidinics: Examples include the A-C eleokanins, E and D grandisins, isoelaeocarpicin and eleocarpenin.
- - Alkaloid type eleokanidine: They present a skeleton base of pirrolo[2,1-f][1,6]naftiridina or 1H-pirano[2,3-g]indolizine. Examples of these alkaloids are the A-C eleokanidins and the D and E eleokanins.
- - Alkaloid type eleocarpine: They present a skeleton base 12H-[1]benzopirano[2,3-g]indolizine, such as eleocarpine, pseudoepiisoeleocarpilin, grandisins C and F, rudrakina, eleocarpilin, aloeleocarpilin, epialoeleocarpilin, epiisoeleocarpilin, isoeleocarpilin, epieleocarpilin.
| Eleokanina A | Isoeleocarpicina | Grandisina G | Eleokanidina A | Eleocarpina |
- Arginine derivatives: Arginine is a precursor of several natural products, such as clavulanic acid, capreomicidine (a hexahidropirimidine), tetrodotoxin and saxitoxins (Saxitoxine, neosaxitoxin, GTX1-7, C1-C4, dcSTX, dcneoSTX, dcGTX 1-4) which present one isH10H- [1.2-cHurry up,
- Verpacamides and peramide are anhydropeptides of arginine and proline:
Porphobilinogen derivatives
Porphobilinogen is the precursor of bilans, porphyrins, corrins, and bilins. Some insect-produced chromophore alkaloids derived from this compound are pterobilin, sarpedobilin, and forcabilin.
Alkaloids derived from lysine
Lysine can be biosynthesized by the diaminopimelate (DAP) pathway in fungi or by the α-aminoadipate (AAA) pathway. During its biosynthesis, metabolites such as picolinic acid and dipicolinic acid can be formed, which are isomers of nicotinic acid and quinolinic acid, respectively.
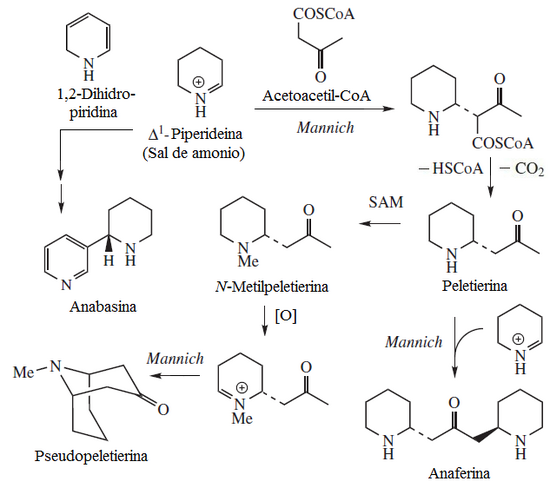
When lysine heterocyclizes, it forms the lysine lactam (Base skeleton: perhydroazepine). This lactam is the basis of the bengamides. Allysine is the transamination product of the amino terminal of lysine. Desmosine comes from this intermediate. Allysine can form pipecolic acid, which is a component of ascomycin and rapamycin. Decarboxylation of lysine produces cadaverine, which by heterocyclization produces Δ1-piperideine. This ring can form several alkaloid systems.
Lysine alkaloids can be classified as cadaverine derivatives and pipecholate derivatives.
- Them derivatives of the are mainly those that come from the incorporation of a piperidina unit to various components, for example:
- - Anaferin, peletierin and pseudopeletierin are derived from acetylation in a manner similar to the biosynthesis of the higrine and the tropane alkaloids.
- - Stenusine is a derivative by condensing with isoleucin. Other examples include evoninic acid, wilfdodic acid, edulinic acid and evonin.
- - Alcaloides piperidinflavonoids' An example is rohitukine, chromtacupines and the derivatives of the captain.
| Schumannioficina | Rohitukina |
- - Alcaloides 1.3-diazepineflavonoids: For example, the north and isoquiledine.
- - Cynolizidal alkaloids: They are formed by two piperidein units. They are found in several members of the Fabaceae family. Typical examples are lupine, lusitanine and castoramine.
- - From peletierin and other derivatives new heterocyclic ring systems are formed, such as cytokine, spinaine, licodine, angustifolina, albine, fawcetimida, poranthrin, cernuine, sufocarpine, cryptopleurine, ormosamine, aphilin, lupanine, retamine, aloperin, baprorin
 |  | |||||
| Esparte | Citisine | Angustifolina | Licodina | Ormosamina | Fawcetidina | Mirionina |
- - Anabasine, astrophylline and anabatin are bis-pyridines from Mannich condensation among other lysonic or nicotin-based piperidins.
- - Lobelanine, lobelin and sedamine are formed by the incorporation of phenylpropanoid derivatives.
- - Pipingrin, nigramid R, pipercyclobutanamide C and the H and I shaves are examples of piperidine alkamides.
- - Indolizidal alkaloids: Castanospermine comes biosynthetically from pipecolic acid. This is condensed with two acetate units by means of Claisen condensations in the same way as in polycets, thus generating the 1-indolizidinona, which is the precursor of many indolizidonic alkaloids. Examples of these alkaloids are castanospermine, swainsonine, pumiliotoxins, lentiginosine, polygonatins and monomorine. Mirmicarinas present an indolizidine skeleton fused with a pyrrol (Pirrolo[2,1,5-cd]indolizine. Indolizidal alkaloids may be fused with aromatic rings (Dibenzo[f,h]pirrolo[1,2-b]isoquinolin) as in the case of ficuseptins, chryptopleurine and thyloforin.
| Indolizina | Pirrolo 2,1,5-cd Indolizine | Dibenzo[f,h]pirrolo[1,2-b]isoquinolina |
- Literary alkaloids: These cyclophanic alkaloids are made up of a cyclozolinic or piperidine cycle from lysine, which are esterified with phenylpropanoid aromatic acids or form ethers between aromatic rings.
- Alkaloids of Nitraria: Gerrit-Jan Koomen and Martin J. Wanner classified alkaloids Nitraria, two of which come exclusively from the lysine: the spiroalcaloids (For example, (+)-nitramine, (-)-isonitramine, (-)-sibirin, nitrabirin, nitrabirin oxide, and sibirinin) and mandiperidynic alkaloids (Schoberine, dehydroschoberine, sibiridine and dihydroschoberine)
- Acromelic acids: Insulated from the fungus Clitocybe acromelalga. They are biogenetically related to kainic acid and domoic acid. What is assured of all these alkaloids is the correlation with glutamic acid. Fusaric acid is condensed in a similar way, only forming a piridine alkaloid.
| Acromelic acid A | Acromelic acid B | kainic acid | Domoic acid | Fusaric acid |
Alkaloids derived from phenylalanine and tyrosine
Phenylalanine and tyrosine are biosynthesized by the shikimic acid pathway, via chorismate, prephenate, and arylpyruvates. Plants and bacteria synthesize both amino acids by separate routes, while animals and fungi can obtain tyrosine by hydroxylation of phenylalanine.
The alkaloids of the tyrosine-phenylalanine amino acids are a very broad and diverse group, which is why they will be classified by their biosynthesis into the following types:
- (a) Alkaloids Securinega
- (b) Alcoholic alkaloids
- (c) Isoquinolynic alkaloids
- (d) Alkaloids mesembrenoids
- (e) Norbeladian alkaloids
- (f) Anhydropeptides of aromatic amino acids
- (g) Alkaloid cytoanics
- (h) Alkaloids of the cycle-DOPA
- (i) Dry alkaloids-DOPAs
Securinega alkaloids
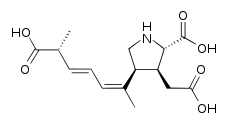
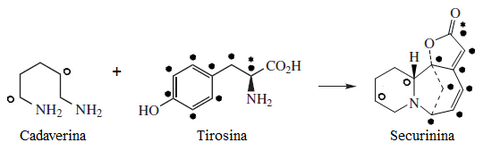
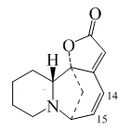
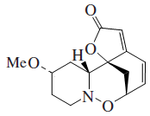
Plants of the genus Securinega produce alkaloids with the base skeleton (6S,11bS)-6,11b-methane-3a,6,11a,11b-tetrahydrofuro[2,3-c]pyrido[1,2-a]azepine. This small group of 30 alkaloids seems to come biosynthetically from tyrosine and lysine, as is the case with securinine. Other examples are the securiniamines, sufruticodin, and the securinols A-D. Phylantidine has the structure methanofuro[2,3-d]pyrido[1,2-b][1,2]oxazocine. Sankawa et al. deduced that securinin may come from a tyrosine molecule and a cadaverine molecule. The lysine, cadaverine, and tyrosine precursors were the ones that showed the highest incorporation. Degradation experiments revealed that [1,5-14C]-cadaverine specifically labeled the piperidine ring of securinin and the radioactivity of DL-tyrosine-[2- 14C] was incorporated into the C-11 carbonyl of the lactone. Experiments with L-tyrosine[U-14C] and L-tyrosine-[3',5'-³H;U-14C] proves that the C6 – C2 fragment is derived from the aromatic ring and the C-2 and C-3 carbons of tyrosine.
| Securinina | Secuamamine D | Filantidina | Securinega suffruticosa |
Heterocycles formed by assembly
- The phenylalanine anhydropeptides form 2.5-pirazinodium-type structures. These metabolites are widely distributed in mushrooms. Anhydropeptides can be diarylic (formed by a peptide condensation of two arilalanins, e.g. picrocceline, albonoursine, phenylalanin anhydride, cyclopenin, viridicatin, Piperafizina B, emeheterone.
| Piperafizina B | Picroccelina B |
Mixed anhydropeptides (formed from an arylalanine and another amino acid). For example, gliotoxin is formed from phenylalanine and serine:
Several fungi (Arachniotus, Aspergillus, Epicoccum) can carry out other condensations and by means of a reverse electrocyclization of an arene epoxide, polycyclic alkaloids such as al aranotine are formed. In this case a second cyclization is carried out, in which a polycyclic compound is formed. Aranotine additionally undergoes reverse electrocyclization from the corresponding arene epoxide to form an oxepine system.
- Other heterocyclic arilalanine assembly systems are celloentered luciferins:
| Coelenteramine | Coelenteramide | Coelenterazine | Medusa. Aequorea victory produces coelenteramide as a product of bioluminescence. |
- Various modified amino acids are constituted by ribosomal and depsid peptides, such as vancomycin, thyrocidine and bauvericin:
| Vancomicina | Tirocidina A | Beauvericina |
Phenethylamines can be incorporated into rings of imidazoles (such as Polycarpine) and oxazoles, such as annuloline, halfordinol, texalin, and texamine:
| Policarpina | Halfordinol |
- Simple phenols from arilalanin degradation can also be assembled without the formation of new carbon skeletons by intramolecular condensation, forming heterocycle systems with two heteroatoms. For example, the luciferin Lampyris noctiluca formed by this mechanism:
Isoquinoline alkaloids
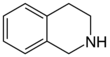
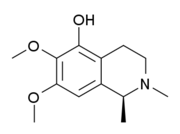
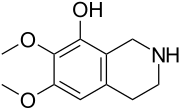
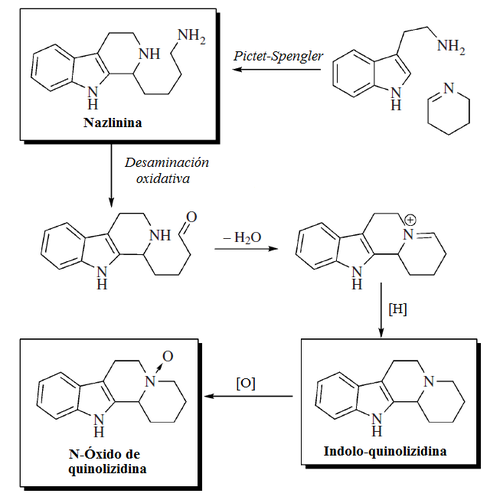
The isoquinoline alkaloids and tetrahydroisoquinoline alkaloids (THIQ) comprise a diverse range of compounds widely distributed mainly in the plant kingdom. It should be noted that these isoquinolines have an alkyl substituent at position 1. Any other substitution pattern suggests another biosynthetic pathway. Biogenetically they can be formed by a Pictet-Spengler reaction of a catecholamine with an aldehyde or an α-ketocarboxylic acid:
| 1,2,3,4-Tetrahydroisoquinolin | THIQ Biosynthesis.
| PeyoteLophophora williamsii)
| Poppy capsule (Papaver somniferum)
| The plants of the genus Berberis produce Berberine-type alkaloids. |
According to the aldehyde used, 4 large families of this type of alkaloids can be recognized:
- (a) The simple isoquinolins, which are formed by condensation of a catecholamine with acetaldehyde, glioxal, piruvato, formaldehyde, etc.
- (b) Bencilisoquinolins, which comprise the wider group of all are formed by condensing a catecholamine with a fenilacetaldehyde.
- (c) Fenetilisoquinolins, which are formed by condensing a catecholamine with a phenylpropanal.
- (d) The unsuccessful alkaloids, where the aldehyde is an iridoid.
- Isoquinolins and tetrahydroisoquinolins simple: Isoquinolin isolates from the plant Spigelia anthelmia (Spigeliaceae). Several examples are anhalamine, anhalinine, iseluxine, calicotomine, laudanosine, coridaldine, salsoline, salsolidine, mimosamicin, renierona, talflavina, crispinas.
| Gigantina | Lofoforina | Anhalamina | Dorianina | Iseluxina | Doryphora sassafras
|
- The group of the saframicine and the renieramicina presents the structure of 6,15-epimino-4H-Ioquino[3,2-b][3]Benzazocina. Examples of these alkaloids are jorunamicins.
- - Bencilchinolinic and bencilquinolynic alkaloids: They are formed by reaction of Pictet-Spengler of a catecholamine with phenylacetaldehydes, coming from the paths of phenylpropanoids. Examples of these alkaloids are papaverin, phylocriptine, phylocriptonine, velucriptine, papaveraldine, and dungeon. The herring and macrostomine, as well as its derivatives additionally have a pyrrolidinio ring attached to the aromatic ring.
| Papaverina | Arenina |
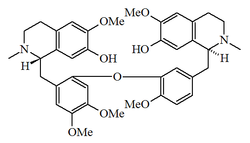
- - Alkaloids bisbencilisoquinolynics: They are produced by couplings of free radicals and are connected by one, two or three ether or biphenyl links. Monomeric units are mainly hydroxylated or metoxylated benzyles. Aporphine may contain aporphine components. This large group of alkaloids can be divided into five categories, according to the Shamma classification:
- (a) Alkaloids that only present coupled arilo groups. From the bark Popowia floorcarpa a group of 7 alkaloids that have links between C-11 and C-11' have been isolated. Some examples are the pisopowetina and the pisopowiaridina.
- (b) Alkaloids containing only one ether link: Ether links are commonly found in C-11 and C-12' carbons, such as dauricin, C-11 and C-10', as in vanuatin, between C-10 and C-7' as malekulatin and ambrinine, C-11 and C-7', as in the nephenine.
- (c) Alkaloids containing a link with an aromatic and one or two ether links. These alkaloids are based on the skeleton of the rodiasine, for example thiacorine.
- (d) Alkaloids containing 2 ether links: The largest simple subgroup containing two ether links has the berbamano skeleton, p. axim. berbamine. Another group of alkaloids are those of the oxiacanno type, such as oxiacantin, for example oxiacantin, which is connected in C-8 and C-7' carbons and between C-12 and C-13'. This group includes the talicberano (C-8 to C-6' and C-11 to C-12'), talidasano (C-8 to C-5¢ and C-11 to C-12'), and talmano (C-7 to C-5' and C-11 to C-12'). All these types contain links between benzyl rings and between the aromatic rings of the tetrahydroisocynolynic component. The hose contains ether links between the benzyl ring of one unit and the aromatic ring of the isochinoline component of the other unit.
| Berbamano | Oxiacantano | Talicberano | Tubocurara |
- (e) Alkaloids with three ether links: These alkaloids include groups of 6'.7-epoxioxiacantano (p. axim.trilobine), 7.8'-epoxioxiacantano, and 8.12'-epoxitubocurarano.
- - Pseudobencilisoquinolynic alkaloids: This term is used to describe an isobenzoquinolin skeleton in which a bencilisoquinolin skeleton in which the aromatic ring is oxygenated in the C-2', C-3' and C-4' carbon. These alkaloids come biogenetically from protoberynic salts by the C8-C8a link excision. Polycarpine, canadaline, Taxilamine and Ledecorina are examples of this type of alkaloids.
- - Eyeline Alkaloids: Alkaloids derived from the cularine (12aS)-2,3,12,12a-Tetrahydro-6,9,10-trimetoxi-1-methyl-1H-[1]benzoxepino[2,3,4-ij]isoquinolin) are tetrahydroisochinolins containing an oxepin or dihydrooxepin merged between C-8 and C-2' carbons. They are formed by an intramolecular oxidative coupling. Examples are composteline, sarcocapnins, gouregine, aristoyagonine, aristocularins and cularine.
- - Drycular Alkaloids: They can be classified into two groups, the B- and C-drycularins. An example of β-secocularins (e.g., sewcularine, and norsecocularin, which are structurally related to alkaloids derived from fenantreno derived from aporphine. C-secocularins (e.g. noyain).
- - Canteen-type alkaloids: They are dimers of a cularine unit and a morphine through a spiro junction. They are found in species of the genus They say.
- - Alkaloid type quetamine: Only three related compounds are known: Quetamine, Drychatamine and Dihydro-ecochetamine. These alkaloids are found in Berberis baluchistanica.
| Cularina | Policarpina | Noyain | Pollution |
- - Pavin type alkaloids: These alkaloids are formed by alternative modes of oxidative cycling of benzoquinolynic precursors. P. example Pavina, Algerian.
- -Alkaloid type amurensin: Amurensina
| Amurensina | Pavina |
- - Benzopirrocolinas: Criptowolidina, criptowolinol, criptowolina.
- - Proaporphin alkaloids: This alkaloid group represents an intermediate stage in the conversion of bencilisoquinolins into certain aporphins. Examples of these alkaloids are the orientalinone, glaziovina, stefarina, mecambrina, pronuciferin.
- - Apornic alkaloids:This large alkaloid group contains the apornic tetracyclic ring system (4)H- dibenzode,g]quinolin)formed by oxidative coupling of a phenol precursor bencilisoquinolin.
Structural variations include:
- (a) simple aporphins and dioxoloaporphins, for example glaucina, boldina, bulbocapnine, nantenin, hernandialin, nuciferin, liriodenine and pukatein. Laurotetamine, lauroscolzine, scoline, magnoflorin. Also included are usually unsaturated derivatives between C-6a and C-7 carbon.
| Aporphine skeleton | Glaucina | Bulbocapnine | Nantenina |
- (b) apoporfinoid type duguenain and pancoridina. Its base skeleton is 9,10-dihydro-5H7H-benzof][1,3]dioxolo[6,7]isoquino[8,1,2-hij][3,1]benzoxazina.
| Pancoridina | Duguena |
- (c) oxoisoaporfinas (p. a.m. menisporfina). They present the structure of 7H-dibenzo(of,h)quinolin
- (d) azafluorantenes (p. axim. rufescin and imelutein). They present the structure of indeno[1,2,3-ij]isoquinolina
- (e) diazafluorants (p. axim. eupolauridina).
- (f) 1-azaoxoaporfinoids (p. axim. sampangina). They present the skeleton of 7H-Nafto[1,2,3-ij][2,7]naftiridina.
- (g) azahomoaporfinas (e.g. dredabine). It presents the structure of benzo[d]-1,3-dioxolo[4,5-g]pyrido[4,3,2-jk][2]benzazepina
| 7H-dibenzo(of,h)quinolin | Indeno[1,2,3-ij]isoquinolina | Eupolauridina | 7H-Nafto[1,2,3-ij][2,7]naftiridina | Benzo[d]-1.3-dioxole[4,5-g]pyrido[4,3,2-jk][2]benzazepina |
- (h) oxidized aporfinoids (e.g. Andesine, chiloenin, santiagonamine).
- (i) thropoloisochinolins (e.g. imerubrina). The base skeleton is 10H-blue[1,2,3-ij]isoquinolin.
- (j) alkaloids type benzo[g]quinolin, for example cleistolin
- (k) lennoxamine-type alkaloids (base skeleton: 5H-[1,3]dioxolo[4,5-h]isoindolo[1,2-b][3]benzazepina)
- (l) alkaloids type 5H-Indeno[1,2-b]piridina, for example oniquina
- (m) compounds in which heterocycle has been opened to give derivatives of fenantrenus, such as taspina.
- (n) alkaloids containing 5-member lactamas rings, such as aristoctamas (such as cepharanone A) and piperolactama. Cephalons tienne as skeleton benzo[f]-1,3-benzodioxolo[6,5,4-cdIdol. Aristolic acid is a derivative of N nitro group oxidation.
| Santiagonamina | 10H-blue[1,2,3-ij]isoquinolin | Benzog]quinolina | 5H-Indeno[1,2-b]piridina | Benzof]-1,3-benzodioxolo[6,5,4-cd]indol | 5H-[1,3]dioxolo[4,5-h]isoindolo[1,2-b][3]benzazepina |
- Bencilisoquinolin-aporfina: They have a single ether link. A typical case is talicarpine.
- - Limalongina
- - Morphinal alkaloids: These alkaloids have as the basis skeleton of morphine. They are formed from the oxidative coupling of a hydroxylated isoquinolynic precursor. Some examples of this group are codeine, salutine, morphine and oripavina.
- - Alkaloid type hasubanano: They form in a way similar to morphine. They isolated from the plant Stephania japonica Hasubanonine, cepharyamine, devalaine, glabradine, limalongine, longanine, longeterin, metaphanine, miersine, periglaucines A-D, prometafanine, prostephabisine, prostephanaberine, runanine, stefadynamine, stefamiersine, stephanine.
- - Alkaloid type 7H- dibenzod.f]azonina: They isolated from the plant Stephania japonica. Protostephanine.
| Hasubanano | 7H- dibenzod.f]azonina |
- Alcaloides benzo[c]phenantridinics:
- - Protoberinic alkaloids: They are tetracyclic alkaloids that have as a protoberine base skeleton. Examples of these alkaloids are anisocycline, palmatin, corlipalmine, discretamine, berlambine, lambertine, choreximine, talifaurine, coptisine, scoulerin, styling,
cavidin, cheilantifolin, corisolidin, coribulbin, coridalidzin, coridalin, coripalmin, corisamine, synactin, capaurimin. This group of alkaloids includes:
- (a) tetrahydroprotoberins, for example tetrahydropalmatins.
- (b) protoberines, such as Berberine;
- (c) Methylated derivatives in position 13 such as coirlin
- (d) dryberries, which have a split ring. Some examples are aobamine, macrantaline and macrantoridine.
- - Alkaloid protoxin type These alkaloids have the skeleton base bis[1,3]benzodioxole[4,5-c:5',6'-g]azecin Protopine, coricavamine, coricavidine, argemexicains A and B, constrictosin, cryptopin, pharmains I - III, muramine, pseudoprotoin.
| bis[1,3]benzodioxolo[4,5-c:5',6'-g]azecina |
- - Alkaloid type roeadina. The Roeadano ([1,3]dioxolo[4,5-h][1,3]dioxolo[7,8]-1H-isocromeno[3,4-a][3]benzazepina) is the base skeleton of these roeadina alkaloids, papaverubins, alpineigenin, glaucamine, zangezurina.
| [1,3]dioxolo[4,5-h][1,3]dioxolo[7,8]-1H-isocromeno[3,4-a][3]benzazepina |
- - Alkaloids spirobencilisoquinolynics: They have as the base skeleton the 1.2-dihydrospiro[2-H-indeno-2,1'-isoquinolin], e.g. lahorin, africanine, coridaine, coristewartin, Smokingicin Smokingilinahyperectine, ocotensin, severzinine, radeanamine, radeanine.
| 1.2-dihydrospiro[2-H-indeno-2,1'-isoquinolin] |
- - Alcaloides benzo[c]fenantridínicos: They may have the basis skeleton [1,3]benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]fenantridine or the bis[1,3]benzodioxolo[5,6-a:4',5'-g]-4H-quinolizine, such as Quelidonine, sanguinarina, coridamine, arnotianamida, corinolamine, arnotianamida, quelilutina, corinolina. Dimeros: Sanguidimerina
| Bis[1,3]benzodioxolo[5,6-a:4',5'-g]-4H- What? | [1,3]benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]fenantridina |
- - Alkaloid type narcein. Bicuculinine, Peshawarina, narceine.
- - Phalidoisocynolynic alkaloids: α-Hidrastine.
- - Fenetylisocynolynic alkaloids
- - Uniform alkaloids They have as skeleton base of the benzo[6,7]ciclohept[1,2,3-ij]isoquinolin, p.axism. kreysigina
| Benzo[6,7]ciclohept[1,2,3-ij]isoquinolin |
- - Alkaloid type colchicin: They are amines of benzo derivatives[a]heptaleno, such as colchicin, O-Metilandrocimbina, autumnalina, alkaloid AM 3, colchiciline, colchifolin, cornigerin, lumicolchicinas, demecolcinona, spicesine.
- - Alkaloid type homoeritrine: The homoeritrinano is a heterocycle with a base structure of [4,5-h]indolo[7a,1-a][2]benzazepine. Examples of these alkaloids are schelhammeridina, erimelantin, erisopinoforin, cefalofortunein, comosine, comosivine, dishomeritrina, fortunein, holidine, homoerisotin, isofelibilidina, lucidininine, felibilina.
| [4,5-h]indolo[7a,1-a][2]benzazepina |
- - Dibenzocicloheptilaminas: These alkaloids have been found in plants of the genus Colchicum and Androcymbium. For example jerusalemine, salimina, alkaloid K4 and colchibiphenyline.
- - Eritrean Alkaloids β-Erytroidine, Erisotramidina. Cephalotaxine presents a structure 4H-cyclopenta[a][1,3]dioxolo[4,5-hI'm sorry.b][3]benzazepina. Harringtonin and isoharringtonin.
| Eritrean |
- Aaptamine-type alkaloids: Aaptamine and its natural congeners are marine alkaloids containing rings 4H-benzoof][1,6]-naftiridina. All aaptamines have been isolated from Demospongiae (Porifera). These alkaloids could be classified into the following groups:
- - Aaptamine derivatives: for example aaptamine and isoaaptamine
- - 1a,3,9-triazapirenos
- - 8H-5,8-diazabenzo[cd]azulenos: For example aaptosin and aaptosamine.
- - Dihouidine type
- -Lamelarine-type alkaloids: The Lamelarinas are a family of pirrolo alkaloids[2,1-a]isoquinolynics related to the nuns, sneezeds and lukianoles. Biosynthetically they are bencilisoquinolynic alkaloids condensed with a phenylpropanoid. More than 30 related compounds and derivatives of the various lamelarins have been isolated. The A-D lamelarines were isolated by Andersen in 1985,
- -Scientist alkaloids: Espiguetidina, dragabine.
- There are various isochinolin oligomers such as repandulin, auroramine (p. axim. pennsylpavina and baluchistanamine) and other alkaloids such as epiberbivaldine and cancentrine.
| Cancentrina | Auroramine | Repandulina |
- Alcaloides de Isopyrum: They are ethers of a THIQ and an aporphine, for example Isopirutaldina, isopitaldine and isotalmidine, talifin and isotalifin.
- - Dry alkaloidsbencilisoquinolynics: They are those in which one of the units of bencilisoquinolin breaks between the C-1 carbon and the α carbon atom. Aldehyde lactams (such as Punjabine), lactam esters (gilgitin, talcamine) or aminoaldehydes (Jhelumina, chenabine). Karakoramine lacks the fragment of lactama, but it has a hydroxymethyl function in C'-aromatic carbon.
| Karakoramina | Punjabine | Jhelimina |
- Aporfinoid numeralssuch as taliaberine, coyhaiquina and hernandaline. Roemeridina and Ourabaine.
- Emethine type alkaloids: These alkaloids form a tetrahydroisocynolin unit from an iridoid aldehyde. The forerunner of these alkaloids is the ipecoside Emetina is the typical alkaloid of this group.
- Alkaloid type ecteinascidine: Trabectidina, ecteinascidines
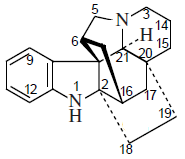
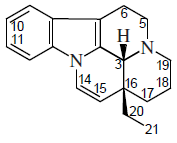
Phenanthroindolizidine and phenanthroquinolizidine alkaloids
- - Fenantroindolizidinics and phenthhinokinolizidinic alkaloids: Tiloforin, Criptopleurine.
Norbeladin and mesembrenoid alkaloids
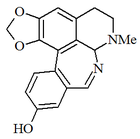
The Amaryllidaceae family produces a group of alkaloids whose precursor is norbeladin, an amine formed by the reduction of the Schiff base formed between protocatechuic aldehyde and tyramine. Norbeladin can couple the two aromatic rings it presents by free radicals. Thus, different structures can be formed according to the pattern of coupling of the rings. Norbeladin, cryptostilline I, cherillin, and nivalidin, galantamine, hemantidine, tazetin, and pancracin. Montanina. Lycorine, Lycorenine. Narcyclasine. Mainly there are skeletons type:
- (a) Licorine
- (b) Crinine
- (c) Galantamine
Mesembrenoid alkaloids are derived from two phenylalanine units with loss of one of the ethanamine side chains, which come from a lobeladin-type intermediate. This group of about 20 alkaloids has three structural types:
- (a) Messembrin type: They have the 3a-phenoctahidro-1 base skeletonHMesembrine, mesembrol, crinafolidine.
- (b) Joubertiamine Type: They form by the rupture of the indol ring, thus forming a linear amine.
- (c) Tortuosamine type: The amine formed in the joubertamine type alkaloids recycles to form a structure type 6-phenyl-5,6,7,8-tetrahydroquinolin.
Dibenzoquinolizine and dibenzoindolizine alkaloids
It constitutes a small family of alkaloids that consist of an aromatic residue from an arylpropane derivative and a condensed cycle, probably formed as cylindrines. They have been isolated from the menispermaceous plants Cocculus hirsutus
| Cohirsina | Cohirsitinine |
Cytochalasins and Pseurotins
Cytochalasins are polyketide alkaloids that consist of an amide of the carboxylic end of the polyketide with the amino group of phenylalanine. Subsequently, this system condenses to form a pyrrolone and subsequently an intramolecular Diels Alder reaction occurs.
Pseurotins are spirofuropyrrolic polyketide alkaloids produced by a precursor polyketide that forms an amide with phenylalanine. Subsequently, the heterocyclization of pyrrole is formed and, finally, spirolactam with subsequent oxidations. Cephalimysins are alkaloids with biosynthesis related to pseurotins.
Cyclo-DOPA alkaloids
Dihydroxyphenylalanine (DOPA) can form 2,3-dihydroindole called cycloDOPA. This compound can form intermediate indoles. When this compound polymerizes, cyclizes, or condenses with cysteine, it forms eumelanins and pheomelanins.
Seco-DOPA alkaloids
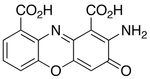
- Betalains. The basis of these alkaloid pigments is the betamic acidwhich is formed by the oxidative excision of the DOPA. When betalamic acid forms magnets with nitrogen of amino acids, they form betalains. They are classified into two types: Betacianins, which are iminous salts of the DOPA cycle, and the betaxanthins, which are magnets with amino acids or biogenic amines. These secondary metabolites of nitrogenated plants act as red and yellow pigments. They are present only in the Caryophyllales taxon except Caryophyllaceae and Molluginaceae (Clement) et al. 1994). In contrast, most other plants have pigments that are anthocyanins (which belong to the group of flavonoids). Betalains and anthocyanins are mutually exclusive, so when betalains are found on a plant, anthocyanins will be absent, and vice versa. Some fungi also present these compounds, called muscaaurines. When instead of forming a heterocyclus of six members (such as betamic acid) it forms one of seven, it is called muscaflavina, and the magnets are called higroaurines.
Alkaloids derived from anthranilate
Anthranilic acid is formed by concerted elimination with a nitrogen addition of glutamine from chorismic acid. This compound is the precursor of multiple secondary metabolites, which can be classified into the following categories:
- (a) Protoalcaloids of anthranilate.
- (b) Fenacious alkaloids
- (c) Mixed polycytes with anthranic acid
- (d) Corporal alkaloids
- (e) Alcoholic alkaloids
- (f) Alkaloids β-carbolinics
- (g) Alkaloids of ergot
- (h) Anhydropeptides of the tripptophan
- (i) Numbers of tripptophan
- (j) Quinine derivatives
- (k) Chetoglobosan alkaloids
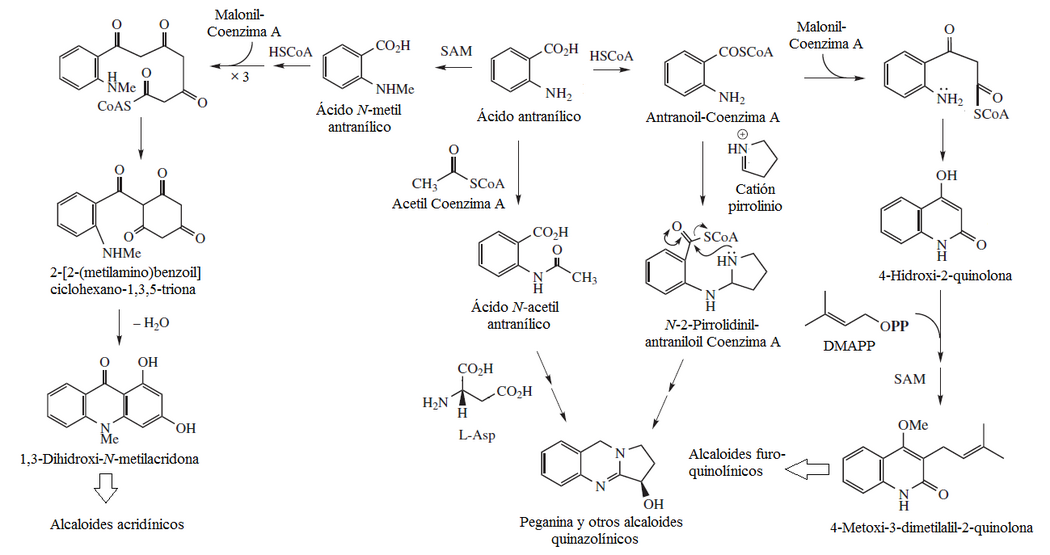
Anthranilate Protoalkaloids
- Protoalcaloids of anthranic acid: Damascus
- Antitranyl acid (Bleniona), lilacinona
- 2HBenzoc]pirano[2,3-h]cinolinas: Necatorina
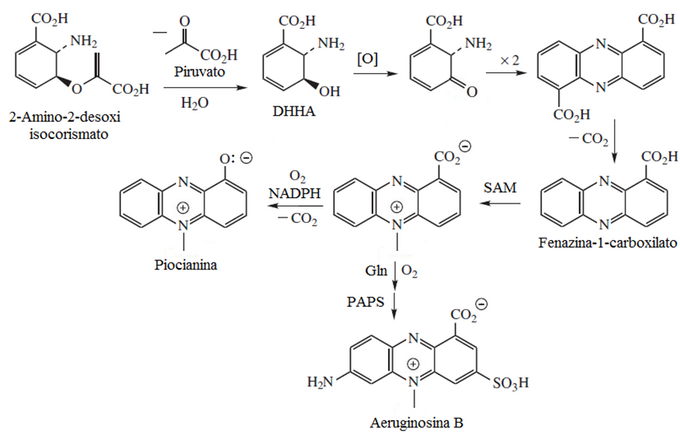
Phenazine alkaloids
- Phenynazinic alkaloids: piocianine, aeruginos, emeralds.
Pyocyanin is a phenazine alkaloid that is one of the many toxins produced and secreted by the Gram-negative bacterium Pseudomonas aeruginosa. Pyocyanin is a blue-colored secondary metabolite with the ability to oxidize and reduce other molecules and thus can kill microbes that compete against P. aeruginosa as well as cells from the lungs of mammals to which P. aeruginosa has infected during cystic fibrosis. Chorismic acid is the precursor of pyocyanin, in which an amino intermediate is formed that, when assembled with two different tautomeric units, generates phenazine-1-carboxylic acid. This compound is a precursor to other phenazines.
- Alkaloids 1,4-benzoxazin-3-ónicos: DIMBOA
Mixed polyketides with anthranilic acid
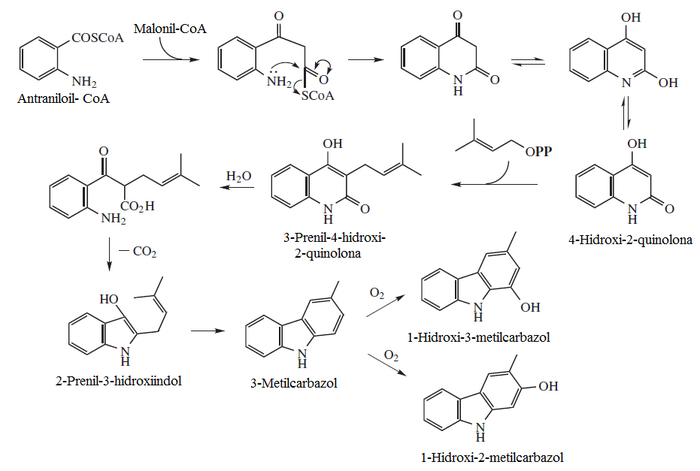
- Quinolons and furo[2,3-b]chinolins: equinopsine, dictamnine, platidesmine, flindersin, eskimianin, cusparina
- Acrydinic alkaloids: xantevodina, 4-hydroxy-2-quinolone, melicopicin, acronicine, routecridone.
- Chindolinic Alkaloids: Criptolepina 10H-Indolo[3,2-b]quinolin (Quindolina), cryptospirolepin, cryptolepinas, isocriptolepin, neocriptolepin, hydroxycriptolepin, cryptoheptin, biscriptolepin, cryptomisrin, and chryptocyndoline.
- Carbazlic alkaloids: murrayafolins, mukonin, koenolina, 3-methylcarbazole, clausins, 1-hydroxy-3-methylcarbazole, mukoeic acid, glycozomine, girinimbinol, furostifolina, clauszolinas, mahanimbinol, murrastifolins, carbazomicin, koen
Quinazoline alkaloids
- Corporal alkaloids and pyrole[2,1-b]quinazolinas: Vasicina, luotonin, febrifuguina, triptoquivalin.Macrorina.
- Dictioquinazoles
- Benzodiazepinic alkaloids: Cyclopenin, viridicatin.
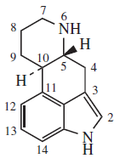
Indole alkaloids
- Methyloxyl, methylbutin, melosatins, methophenyl, methodox, methylxine.
- Pyrimidilindical Alkaloids: Meridianins, psamopemines, isomeridianins
- Pyrroloindlic alkaloids: They are formed by heterocyclization of the tritamine to form 1.8-dihydropole[2,3-b]indoles such as Quimonantina, fisostigmina, flustramines.
| 1.8-dihydropirrole[2,3-b]indol |
- Necatarones
| Necatarona |
β-carboline alkaloids
- Simple β-carbolins: They are formed by formaldehyde condensation, pyruvate, acetaldehyde, etc.
β--Carboline, Harmano, eleagnine, harmine, harmaline, Pseudofrinamines A, 5-Bromo-6-hydroxy-β-carboline, pseudofrinaminol, perlolyrine, borrerine, eudystomins, cantin-6-one, infractins, flazin, cantinones.
- Picrasidins: Bis-β-carbolins produced by tritamine condensation with adipaldehyde.
- Eudistomins: Alkaloid group that condense with cysteine isolated from tunicates:
- Merged carbolins with anthranilate: Evodiamina
| β-Carbolina | Harmina | Eudistomina C | Evodiamine |
- Alkaloids pyrrolo[4,3,2-ofCynolinics: Indoloquinones with intramolecular cycling of an imino link that form structures of pirrolo[4,3,2-ofChenolin. Examples: Makaluvaminas and micearrubinas. Bloodguinones have 2 other merged rings, just like hetatopodins
| Pirrolo[4,3,2-ofChenolin,
makaluvamine base structure | Sanguinona A | Hematopodina |
- Infractopicrines: Pentacyclic Alkaloids isolated from Cortinarius infracta.
| Infractopicrin: |
- Indual alkaloids of Nitraria: They are formed by the condensation of a hydrolyzed piperidein ring from the lysine (Alkaloids β-carbolinmonopiperideinics) or with two piperidein units (Alkaloids β-carbolindipiperideinics). Examples of these alkaloids are nazlinine, schobericin, komaroidin, komavinas, nitrarin, nitramidine, nitraricine, nitrarizine, tangutorine, nitrarain, komarovina, komarovicin, komarovicin, komarovidina, isokomarovina, nitramarina
- Strictosidinic alkaloids: Strictosidine
- Camptotecin type alkaloids: They present rings of (1H4H,12H)-pirane[3',4':6,7]indolizino[1,2-bChenoline like Rubescina, camptotecina.
| 1H4H,12H)-pirane[3',4':6,7]indolizino[1,2-b]quinolina |
- Indoloquinolizidinic alkaloids: Angustin, Deplanchein
| Angustina |
- Alkali type Corynanthe: Geissosquizina, sitsirikina, Ocrolifuanins, Usambarinas
| Geissosquizina | Ocrolifuanine A |
- Ajmalicin-type alkaloids: Ajmalicin, oxayohimbano, corinantein
| Corinano | Oxayohimbano | Ajmalicina |
- Oxyxinal alkaloids: Rincofiline, Formosanina
- Alkaloids Gelsemium: Gelsedina, gelsemina
- Alcaloides yohimbinoides: Yohimbina, reserpina, Alstonilina, rescinamine.
- Alkaloids type Akuammilina: Akuammilina, equitamine, erypine, aspidodasicarpine, alstofiline, narelina,
| Akuamilano | Macrolina |
- Sarpagine-type alkaloids: sarpagine, vobasin, gardneramine, evatamine, erythsine and koumine.
| Sarpagano | Akuamidina | Ervatamine |
- Ajmalan alkaloids: Perakina, Raucaffrinolina, ajmalina
- Pleiocarpamine type alkaloids: Pleiocarpamine
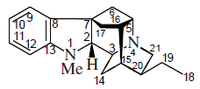
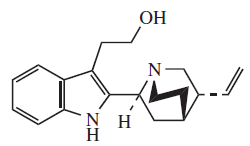
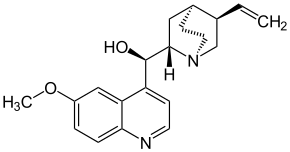
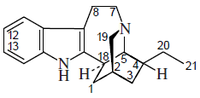
- Five-type alkaloids: This alkaloid group includes antimalaric quinine, and contain a quinuclidine ring. This group is classified into two groups:
Quinine, cinchonamine (a) the cinchonamine group, derived from a corinanthine-type precursor by fission of N-4 to the C-5 bond, and by union of N-4 to C-17, (b) the quinine group, which contains a quinoline ring generated by fusion of the 2,7 bonds followed by an N-1 to C-5 fusion of the cinchonaminal.
| Five | Quinina |
- Stricnine-type alkaloids: They are formed from akuammicin and suffer a later aldialic condensation with a coenzyme A acetyl unit. Some examples are Estricnidine, prekuamicin, brucina, estemadenine, estricnine.
- Condilofolanic alkaloids: Condilocarpine, goniomine
- Goniomine-type alkaloids: Goniomine
- Dryedy alkaloids: Secodine and andranginin.
- Ibogamine alkaloids: It consists of a series of alkaloids that come from the fission of the prekuamicin, where the stemadenine is formed and by reaction of Dielsa-Alder a new ring of ibogamine is formed.
Examples of these alkaloids are Cataranthine, coronaridine, tabernoxidine.
| Ibogamine |
- Alkaloids aspidospermidinics: The skeleton of the aspidospermidian alkaloids is formed by the cyclization of dehydrosecodine, obtained from a precursor of dehydrosecodine.
Alkaloids in this group include the following structural variants:
- (a) anilinoacrylic alkaloids, such as tabersonin, which contains the metoxycarbonyl group in carbon 16. The two carbon substitutes in position 20 can be a simple ethyl group, or can be functionalized.
(b) alkaloids lacking the carbon 16 methoxycarbonyl group, as in aspidospermine. C-18 and C-19 can be an ethyl group or C-18, which can be functionalized.
(c) alkaloids containing an ether or lactone bridge between C-18 and C-21; (d) alkaloids containing an ether or lactone bridge between C-18 and C-15; (e) alkaloids containing a lactone bridge between C-18 and C-17, and a dihydro-1,4-oxazine ring between N-1 and C-12, as in obscurinervidin:
| Obscurinervidina |
(f) alkaloids containing an additional bond between C-18 and C-2, as in venalstonin;
| Venalstonin |
(g) alkaloids containing an additional bond between C-19 and C-2, as in vindolinine;
| Vindolin |
(h) the quebrachamine group, which is derived from the fission of the 7,21 bond. These alkaloids may have lost the C-16 methoxycarbonyl group, as in quebrachamine, or it may be retained, as in vincadine. Examples of these alkaloids are kopsiyunanins.
| (+)-Quebrachamina | Vincadina |
(i) Dimers such as vinblastine, vinorebine and vincristine. (V. Vinca alkaloids) (j) various alkaloids formed by a wide variety of processes, e.g. Ahem. aspidodispermine, banucin, vincatine, razinilama, trichophyllin, meloscin, melonin, goniomitin
- Kopsan alkaloids: Kopsina, Fruticosine
- Alkaloids type lapidilectin and lundurine.
| Lapidilectina B | Lundurina B |
- Pandoline alkaloids: Pandolina, Cleavamina, Pandina, Iboxifilina, Ibofilidine
- Pyridocarbazlic alkaloids: These alkaloids are formed by the excision of the estemadenin and by cycling on the ring of indol. Examples of these compounds are Olivacina, Elipticina, guatambuina, janetina
- Ulein-dasicarpidan alkaloids: Ngouniensin, vallesamine, uleine
- Conofilidine-type alkaloids.
- Alkaloids Eburna: Eburnamina, cuanzina, Equizozigina, Andrangina and Vallesamidina
The skeleton of these alkaloids is generated by rearrangement of the aspidospermidine system, by migration of carbon 21 from carbon 7 to carbon 2, fission of bond 2,16 and by union of C-16 to N-1. These alkaloids can be classified into: (a) Vincamine and its derivatives, which retain the methoxycarbonyl group; (b) alkaloids such as eburnamine and eburnamenine, which have a loss of the ester group at C-22; (c) some derivatives where C-18 or C-19 are oxidized like quanzine; (d) The schizozygine group, which contains an additional bond between C-2 and C-18; (e) Related alkaloids andrangine and vallesamidine, where C-21 has migrated to C-2.
| Eburnamenine |
- Alkaloid type manzamine
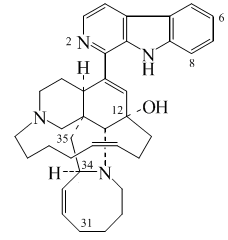
Ergot alkaloids and other indoleterpenes
- Ergolin alkaloids: Chanoclavins, elimoclavine, cyclopiazonic acid, ergocristin, ergometrine, ergoclavine, clavictic acid, lysergy acid, ergine, agroclavine, paspalic acid, elimoclavine, ergocornin.
- Alkaloid type Lolitrem: Lolitrem
- Non-dulhispanic acid
- Lolicinas
- Alkaloids indolo[2,3-aCarbazlics:
- Hapalindoles: Cyanophyta's isolated preniled idols.
| Hapalindol A |
- Alkaloids AristoteliaAristotelina
| Aristotelina |
- Alkaloids Borreria: Borrecapine, borrelina, borreverina.
| Borrecapina |
- Alkaloid type notoamine
Tryptophan dimers
- Alkaloid type Caulerpa: Caulerpina
- Alkaloids indoloflavonoids: Lotthanongina
- Alkaloids type arciriaflavina and staurosporin
- Butforamidines
- Asterriquinonas
Alkaloids derived from kynurenine
Kynurenine, Xanthurenic acid, kynurenic acid, Orellanine
- Phenoxazineic alkaloids: Cinabarin, cinnabarin acid, tramesanguina, polystictin, phenoxazone, α-aminophenoxazone. They are typical mushroom alkaloids Pycnoporus.
- Dercytine-type alkaloids and cyclodercitin. Kuanoniaminas.
Tryptophan anhydropeptides
- Anhydropeptides of tryptophan, spirodesmines, imidazoloids and Almazolonas: spirodesmines, Almazolones, equinuline, brevianamides, roquefortin, verruculgen, oxaline, indolactamas, sporidesmines, aranotin, hamacantins, espongoins, topsazixo, tardyna
- Morfollinic indolyl alkaloids: oxazinins..
- Alkaloid type 4-fenil-[2,7]-naftiridina: Some examples are lofocladines.
Ketoglobosan alkaloids
- Chetoglobosan and related skeletons: Polycyte alkaloids with a stereotyphal unit and modified by a diels-Alder cycladdition. Includes Quetoglobinins, prochetoglobinins, isochetoglobinins, penocalasins and cytoglobinins.
- Alanditripinona, Alantrifenona, Alantripineno, Alantrileunona
Alkaloids derived from histidine
- Idiocese alkaloids
- - Alkaloids derived from histidine: Histamine, uroconic acid, murexin, diphetamine, ergotionine
- - Alkaloids of the Jaborandi: For example pilocarpine, dolicoteline and pilosine
- Clatrodinic alkaloids
- - Clatrodina type friends
- - 6.8-dihidropirimido[4,5-c[3.2-e]azepinas: Latonduins
- - Agelaspongin-type alkaloids and fakeline
- - Alkaloid type agelastatin
- - Numbers of clotrodines:
- (a) Alkaloid type agelamida
- (b) Alkaloid type ageliferina
- (c) Alkaloid type sceptrine
- (d) Alkaloid type stilisazol
- (e) Macrophakeline:: For example palauamine, masadine, axinelamine and the styleguanidine.
Alkaloids derived from branched-chain amino acids
- Pyrosles: Tenuazonic acid
- Pirazinas: Asperegylic acid, flavacol, pulcherymic acid, neoasperectolic acid
- Sarcodoninas
Genalkaloids
Genalkaloids —or alkaloid amino oxides— are derived by oxidation from alkaloids that contain the group R=(NO)-R, where nitrogen has a valence V, as opposed to normal alkaloids, where it is trivalent (R =N-R). Its action is the same as that of the alkaloid from which they come, but it is slower. They are named by adding the prefix gen- to the name of the alkaloid.
Some genoalkaloids are found in nature, such as geneserine (derived from the alkaloid eserine (physostigmine)) present in the Calabar bean, as well as sarcoviolins and sarcodonins.
Pseudoalkaloids
Pseudoalkaloids are secondary metabolites that have a nitrogen atom incorporated as ammonia into a biomolecule such as a terpene, polyketide, fatty acid, or shikimic acid derivative.
Chemotaxonomy
Alkaloids have been found in gymnosperms such as Cycas, Pinus, Ephedra and Podocarpus. Alkaloids have been isolated from many living systems other than plants, including bacteria, cnidarians, sponges, true fungi, vertebrates, and arthropods.
Alkaloids of bacteria and archaea
| Phylum Proteobacters | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Beta Proteobacteria | Neisseriales | Family Neisseriaceae:
|
|
| Burkholderiales | Family Oxalobacteraceae:
Family Burkholderiaceae:
| Toxoflavina | |
| Gamma Proteobacteria | Pseudomonadales | Pseudomonadaceae family:
|
|
| Enterobacterials | Family Enterobacteriaceae: Prodigiosine-type TrispiralsSerratia) |
| |
| Delta Proteobacteria | Myxococcales | Family Polyangiaceae:
| Epotilonas A (R = H) and B (R = CH3) |
| Phylum Cyanobacteria | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Cyanophyceae | Nostocal | Family Nostocaceae:
| Anatoxin A |
| Oscillatorials | Noctuoid family:
Family Phormidiaceae:
| Saxitoxin | |
| Stigonematales | Family Mastigocladaceae:
| Hapalindol A | |
| Phylum Actinobacteria | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Actinobacteria | Actinomycetales | Pseudonocardiaceae family:
Family Actinomycetaceae:
Family Streptomycetaceae: Various antibiotic alkaloids have been isolated from various species of Streptomycessuch as:
Family Micromonosporaceae:
Family Nocardiaceae:
Family Actinoplanaceae:
| Yatakemycina Mitomycin A Duocarmycin A Staurosporin Olivoretina E |
Alkaloids isolated from algae and protozoa
| Phylum Dinoflagellata | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Dinophyceae | Suessiales | Family Symbiodiniaceae
| Simbioimina |
| Phylum Rhodophyta | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Florideophyceae | Ceramies | Family Rhodomelaceae:
Family Delesseriaceae:
| Almalo |
| Rhodophyceae | Ceramies | Family Delesseriaceae:
Family Rhodomelaceae:
| Lofocladina A |
| Phylum Chlorophyta | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Bryopsidophyceae | Bryopsidales | Family Caulerpaceae:
| Caplergenic acid (caulerpina) |
| Ulvophyceae | Cladophorales | Cladophoraceae Family:
| |
Alkaloids isolated from fungi
| Phylum Mycetozoa | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Myxogastria | Trichiida | Family Arcyriaceae:
| Arciriaflavina A |
| Liceida | Family Reticulariaceae:
| ||
| Physarales | Family Physaraceae:
Family Didymiaceae:
| ||
| Phylum Ascomycota | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Saccharomycetes | Saccharomycetals | Family Saccharomycetaceae:
| |
| Sedis | Sedis | Pseudeurotiaceae Family:
| |
| Dothideomycetes | Plenary | Family Pleosporaceae:
Family Corynesporascaceae
Family Sedis
| |
| Sordariomycetes | Hypocres | Clavicipitaceae Family:
Family Ophiocordycipitaceae:
Family Hypocreaceae
| Lolitrem B Lolina |
| Sordariales | Family Chaetomiaceae:
| Chetoglobosine A | |
| Xylariales | Xylariaceae Family:
| Citocalasina A | |
| Magnaporthales | Family Magnaportaceae:
| ||
| Eurotiomycetes | Chaetothyriales | Family Herpotrichiellaceae:
| |
| Euros | Family Trichocomaceae:
Family Nectriaceae:
Family Elaphomycetaceae:
| Asperegylic acid Gliotoxin Talarotoxine | |
| Onygenales | Family Onygenaceae:
Family Gymnoascaceae:
| ||
| Arthoniomycetes | Arthoniales | Family Roccellaceae:
| α-Aminoorcein |
| Phylum Basidiomycota | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Agaricomycetes | Cantharellales | Family Ceratobasidiaceae:
| Eslaframina |
| Phallales | Family Phallaceae:
| ||
| Russulales | Russulaceae Family:
Family Hericiaceae:
| Necatorine | |
| Agaricales | Family Cortinariaceae:
Family Tricholomataceae:
Family Agaricaceae:
Family Hymenogasteraceae:
Amanitaceae Family:
Family Hygrophoraceae:
| Infractopicrin Muscle (Panterina) Psilocy | |
| Cookies | Family Boletaceae:
Family Suillaceae:
Family Mycenaceae:
| Sanguinona A Hematopodina Paquidermina | |
| Polyporales | Family Meripilaceae:
Family Fomitopsidaceae:
Family Polyporaceae:
| Cinnabarin acid | |
| Thelephorales | Family Bankeraceae:
| ||
Alkaloids isolated from plants
Alkaloids are widely distributed in the plant kingdom (25% of plants contain alkaloids) and in some species their concentration can reach 10% (flowers). In the families Amaryllidaceae, Fabaceae, Liliaceae, Papaveraceae and Rutaceae, alkaloids have great chemotaxonomic value. The Solanaceae family is rich in alkaloids, but with differences at the genus level. Thus, in tobacco (Nicotiana) there are derivatives of pyridine (nicotine), in Solanum (potato, eggplant, tomato) there are spirosolane pseudoalkaloids (tomatine) and Datura, Hyoscyamus, Atropa and Scopolia contain tropane derivatives: hyoscyamine, atropine, etc.
Pyrrolizidines are found primarily in the families Compositae, Boraginaceae, Leguminosae, and Apocynaceae. The producing genera of these alkaloids are distributed in different regions and climates and could represent up to 3% of flowering plants. Some contain only one class of pyrrolizidine, but most contain between five and eight classes. The content varies with each species, but it can be a significant percentage of the dry weight. The highest concentration is found in the roots and is higher in young leaves, inflorescences and flower buds than in older leaves. In some species, high concentrations were found in seeds, which implies a risk in cases where these seeds are used for human consumption. There are N-oxides of these alkaloids, which are more soluble in water and are more easily transported within the plant.
| Phylum Pteridophyta | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Pteridopsy | Polypodiales | Family Davalliaceae:
| Davaliósidos |
| Phyllum Lycopodiophyta | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Lycopodiopsy | Lycopodials | Family Lycopodiaceae::
Family Huperziaceae:
| Licodina |
| Phyllum Pinophyta | |||
| Class | Order | Families | Example |
|---|---|---|---|
| Pinopsida | Pinales | Family Pinaceae:
Family Cephalotaxaceae:
| Harringtonina. |
| Phyllum Gnetophyta | |||
| Class | Order | Families | Example |
|---|---|---|---|
| Gnetopsida | Ephedrales | Ephedraceae Family:
| Ephedrine. |
Angiosperm plants:
- Monocotyledonae (Liliopsyda;Cronquist): Many alkaloids from monocotyleon originate in phenylalanine and are exclusive to this taxonomic group, such as fenetylisocynolynic alkaloids and derivatives of norbeladine.
| Phyllum Magnolophyta: Monocots Class (Clase Liliopsida; Cronquist) | ||
| Order | Families | Examples |
|---|---|---|
| Lilies | Family Liliaceae:
Family Colchicaceae:
Family Melanthiaceae:
| Schelhameridina Jervina |
| Shoot them. | Family Amaryllidaceae:
Family Asparagaceae:
Family Xanthorrhoeaceae:
| Galantamine |
| Arecales | Family Arecaceae:
| Arecolina |
| Poales | Family Poaceae:
| Telepogina |
| Pandanales | Stemonaceae Family:
| Tuberoestemonine |
| Orchidales | Family Orchidaceae:
| trans-Dendrocrisanine. |
| Alismatales | Family Zosteraceae:
Family Araceae:
| Irnidine |
| Goals | Familia Dioscoreaceae:
| Godcorina |
| Phyllum Magnolophyta: Clado Eudicots (Clase Magnolopsida; Cronquist) | |||
| Subclase (Cronquist) | Orden | Familias | Ejemplos |
|---|---|---|---|
| Magnoliidae | Nymphaeales | Familia Nymphaeaceae:
|
Nufaridina |
| Piperales | Familia Piperaceae:
Familia Saururaceae:
|
Piperina | |
| Aristolochiales | Familia Aristolochiaceae:
|
Ácido aristolóquico | |
| Laurales | Familia Calycanthaceae:
Familia Hernandiaceae:
Familia Atherospermataceae
|
Vanuatina | |
| Magnoliales | Familia Magnoliaceae:
Familia Annonaceae:
Familia Himantandraceae:
Familia Myristicaceae:
|
Eupolauridina Espiguetidina Oniquina | |
| Ranunculidae (Cronquist) | Ranunculales | Familia Ranunculaceae:
Familia Berberidaceae:
Familia Menispermaceae:
|
Aconitina Berberina Menisporfina Rufescina |
| Papaverales | Familia Papaveraceae (incluye ant. fam. Fumariaceae):
|
Papaverina Morfina Protopina | |
| Caryophyllidae (Cronquist): Todas producen betalaínas en lugar de antocianinas. | Caryophyllales | Familia Cactaceae:
Familia Ancistrocladaceae:
Familia Amaranthaceae:
Familia Polygonaceae:
|
Anhalamina |
| Dileniidae (Cronquist) | Cucurbitales | Familia Cucurbitaceae:
|
L-β-Pirazol-1-ilalanina |
| Ericales | Familia Theaceae:
Familia Lecythidaceae:
Familia Actinidiaceae
|
||
| Brassicales | Familia Caricaceae
Familia Capparaceae:
Familia Brassicaceae:
Familia Salvadoraceae:
|
Lunarina Ascorbígeno | |
| Malvales | Familia Malvaceae:
|
||
| Hamamelidae | Daphniphyllales | Familia Daphniphyllaceae:
|
|
| Fagales | Familia Fagaceae:
Familia Casuarinaceae:
|
||
| Rosidae (Cronquist) | Malpighiales | Familia Phyllanthaceae:
Familia Salicaceae:
Familia Rhizophoraceae:
|
Securinina Astrofilina Gerrardina |
| Celastrales | Familia Celastraceae
|
Macrolactonas del ácido evonínico | |
| Fabales | Familia Fabaceae:
|
Ormosamina Angustifolina Latirina Fisostigmina Trigonelina Gramodendrina | |
| Proteales | Familia Elaeagnaceae:
Familia Nelumbonaceae:
Familia Proteaceae
|
Neferina Chalcostrobamina | |
| Rosales | Familia Moraceae:
Familia Urticaceae:
Familia Crassulaceae
|
Broussonetinina A Criptopleurina Sedacriptina | |
| Cornales | Familia Nyssaceae:
Familia Hydrangeaceae:
Familia Alangiaceae:
|
Camptotecina | |
| Zygophyllales | Familia Zygophyllaceae:
|
Harmina | |
| Oxalidales | Familia Elaeocarpaceae:
|
Aristotelina Eleocarpina | |
| Sapindales | Familia Rutaceae:
Familia Meliaceae:
Familia Simaroubaceae:
Familia Nitrariaceae:
|
Pilocarpina Cusparina Dictamnina Rutacridona Rohitukina | |
| Euphorbiales | Familia Buxaceae:
Familia Euphorbiaceae:
|
Astrocasina Julocrotina | |
| Myrtales | Familia Lythraceae:
Familia Combretaceae:
Familia Vochysiaceae
|
Soneratina A | |
| Apiales | Familia Apiaceae:
Familia Vochysiaceae:
|
Coniina | |
| Asteridae (Cronquist): | Solanales | Familia Convolvulaceae:
Familia Solanaceae:
|
Cocaína α-Solanina Nicotina Anaferina |
| Asterales | Familia Asteraceae:
Familia Campanulaceae:
|
Lobelina Cotuzinas | |
| Lamiales | Familia Boraginaceae:
Familia Acanthaceae:
Familia Bignoniaceae:
Familia lamiaceae:
|
Estaquidrina | |
| Gentianales | Familia Loganiaceae:
Familia Gentianaceae:
Familia Apocynaceae: Esta familia representa uno de los grupos más importantes en lo que a diversidad de alcaloides concierne:
Familia Rubiaceae:
Familia Gelsemiaceae:
|
Cafeína Reserpina Ibogaína Elipticina Conofilidina Vinblastina | |
Alkaloids isolated from animals
| Phylum Porifera | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Demospongiae | Hadromerida | Family Suberitidae:
Family Trachycladidae
| Aaptamine |
| Haploscleride | Petrosiidae Family:
Callyspongiidae Family:
Chalinidae Family:
Family Niphatidae:
Family Chalinidae
| Ingenamine Xestospongina A Manzamina A | |
| Poeciloscleride | Myxillidae Family:
Acarnidae Family:
Hymedesmiidae
| Dilemaona A | |
| Astrophorida | Ancorinidae Family:
Family Coppatidae
| Acorinósido A | |
| Agelasida | Agelasidae Family:
Astroscleridae Family:
| Palauamina | |
| Halichondrida | Axinellidae Family:
Family Halichondriidae:
| ||
| Dictyoceratida | Thorectidae Family:
Irciniidae Family:
Spongiidae Family
| ||
| Poeciloscleride | Chondropsidae Family:
Crambeidae Family:
| ||
| Choristida | Ancorinidae Family:
| ||
| Dendroceratida | Darwinellidae Family:
| ||
| Lithistida | Family Theonellidae:
| ||
| Verongida | Pseudoceratinidae family:
| ||
| Calcárea | Clathrinida | Clathrinidae Family (Naamidines, polyandrocarpamines, leucetamine; Leucetta) | |
| Phylum Cnidaria | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Anthozoa | Alcyonacea | Xeniidae Family:
| Cespitulactama D |
| Pennatulacea | Renillidae Family:
| Coelenteracin | |
| Actiniaria | Stichodactylidae
| ||
| Scleractinia | Dendrophylliidae Family:
Acroporidae family
| ||
| Antipatharia | Family Antipathidae
| ||
| Zoanthidea | Family Parazoanthidae
| ||
| Stolonifera | Clavulariidae family
| ||
| Phylum Bryozoa | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Gymnolaemata | Cheilostomata | Flustridae Family:
| Chartelina A |
| Ctenostomata | Family Vesiculariidae
| ||
| Phylum Platyhelminthes | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Turbellaria | Polycladida | Euryleptidae Family:
| Villatamina A |
| Phylum Mollusca | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Bivalvia | Mytiloida | Family Mytilidae
| General structure of oxazinins |
| Pterior | Family Pinnidae
| Pinnaic acid | |
| Gastropda | Opisthobranchia | Gymnodorididae Family:
Velutinidae Family:
Aglajidae family
| |
| Sorbeoconcha | Muricidae Family:
Family Babyloniidae:
| Surugatoxin | |
| Vetigastropoda | Trochidae family
| ||
| Anaspide | Family Aplysiidae
| ||
| Nudibranchia | Family Discodorididae
Family Proctonotidae
| Janolusimida | |
| Phylum Arthropoda | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Insect | Diptera | Family Drosophilidae:
Tephritidae
| |
| Coleoptera | Coccinellidae Family:
Staphylinidae Family: Pyrrolidinic alkaloids (Estenusins; Stenus) | Estenusina Hipodamine | |
| Himenoptera | Crabronidae Family:
Formicidae Family:
Family Vespidae:
| ||
| Lepidoptera | Family Pieridae:
Gelechiidae Family:
Family Arctiidae
Family Nymphalidae
Family Saturniidae
| ||
| Diplopoda | Glomerida | Glomeridae Family:
| Glomerin |
| Polyzoniida | Family Polyzonidae:
| Nitropolizonamina | |
| Phylum Echinodermata | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Crinoid | Comatulida | Himerometridae family
| Homoaerotionine |
| Stelleroida | Ophiurida | Family Ophiocomidae
| Ofiuroidina |
| Phylum Chordata | |||
| Class | Order | Families | Examples |
|---|---|---|---|
| Ascidiacea | Enterogona | Polyclinidae Family:
Clavelinidae Family:
Family Perophoridae:
Pseudodistomidae Family:
| Haouaminas Ascidiatiazona B |
| Phlebobranchia | Family Diazonidae
| Diazonamide A | |
| Aplousobrachia | Family Didemnidae:
Family Polycitoridae
| Ascididemine Riterazina M | |
| Stolidobranchia | Styelidae Family:
| Policarpina | |
| Amphibia | Caudata | Salamandridae Family:
| Samandarina |
| Anura | Dendrobatidae Family:
| Histrionicotoxine 283A | |
| Birds | Cuculiforms | Musophagidae Family:
| Turacina |
| Passeriformes | Pachycephalidae Family:
| Batraciotoxin | |
| Mammalia | Carnivora | Mustelidae Family:
Family Mephitidae:
Family Canidae
| 2-(Mercaptomethyl)quinolin |
Contenido relacionado
Achromatic spindle
Joule per mole
Cell (disambiguation)