Alkaline
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Group | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Grupo nomenclatura CAS | IA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Elements | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
The alkali metals or simply alkali metals (from Arabic, alqali) are these six chemical elements: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs) and francium (Fr). These elements, along with hydrogen (which is a gas), make up group 1 found in the s-block of the periodic table.
All alkali metals have their outermost electron in an s-orbital, this shared electron configuration results in their having very similar characteristic properties. In fact, the alkali metals provide the best example of similar group patterns in their periodic table properties, with elements exhibiting characteristic homologous behavior. This family of elements is also known by the lithium family as this is its first element.
The alkali metals are shiny, soft metals, highly reactive at standard temperature and pressure, and readily lose their outermost electron to form cations with +1 charge. All can be easily cut with a knife due to their smoothness, exposing a shiny surface that tarnishes quickly in air due to oxidation by atmospheric moisture and oxygen (and in the case of lithium, nitrogen). Due to their high reactivity they must be stored under oil to avoid reaction with air and they occur naturally only in salts and never as free elements. Cesium, the fifth alkali metal, is the most reactive of all metals. All alkali metals react with water, and the heavier alkali metals react more vigorously than the lighter ones.
All of the alkali metals discovered occur in nature as their compounds: in order of abundance, sodium is the most abundant, followed by potassium, lithium, rubidium, cesium, and finally francium, which is very rare due to its radioactivity extremely high; Francium occurs only in small traces in nature as an intermediate step in some dark side branches of natural decay chains. Experiments have been carried out to attempt the synthesis of a ununene (Uue), which is probably the next member of the group; none were successful. However, ununenium may not be an alkali metal due to relativistic effects, which are predicted to have a large influence on the chemical properties of superheavy elements; even if it turns out to be an alkali metal, it is expected to have some differences in physical and chemical properties from its lighter counterparts.
Most of the alkali metals have many different applications. One of the best known applications of the pure elements is the use of rubidium and cesium in atomic clocks, of which cesium atomic clocks form the basis of the second. A common application of sodium compounds is the sodium vapor lamp, which emits light very efficiently. Table salt, or sodium chloride, has been used since ancient times. Lithium is used as a psychiatric medication and as an anode in lithium batteries. Sodium and potassium are also essential elements, having important biological functions as electrolytes, and while the other alkali metals are not essential, they also have various effects on the body, both beneficial and detrimental.
History
Sodium compounds have been known since antiquity; Salt (sodium chloride) has been an important product in human activities, for example the word salary, in reference to Salarium, the money paid to Roman soldiers for the purchase of salt. Although potash has been used since ancient times, it was not understood for most of its history to be a fundamentally different substance from the mineral salts of sodium. Georg Ernst Stahl obtained experimental evidence leading him to suggest the fundamental difference of the sodium and potassium salts in 1702, and Henri-Louis Duhamel du Monceau was able to demonstrate this difference in 1736. The exact chemical composition of potassium and potassium compounds Sodium, and the chemical element status of potassium and sodium, was not known at the time, so Antoine Lavoisier did not include either alkali in his list of chemical elements in 1789.
Pure potassium was first isolated in 1807 in England by Humphry Davy, who derived it from caustic potash (KOH, potassium hydroxide) by using electrolysis of the molten salt with the newly invented voltaic cell. Previous attempts at electrolysis of the aqueous salt were unsuccessful due to the extreme reactivity of the potassium. Potassium was the first metal to be isolated by electrolysis. Later that year, Davy reported the extraction of sodium from the substance similar to caustic soda (NaOH, lye) by a similar technique, showing that the elements and, therefore, the salts are different.
Petalite (Li Al Si4 O10) was discovered in 1800 by the Brazilian chemist José Bonifácio de Andrada in a mine on the island of Utö, Sweden. However, it was not until 1817 that Johan August Arfwedson, working in the laboratory of chemist Jöns Jacob Berzelius, detected the presence of a new element while analyzing the mineral petalite. He noted that this new element formed compounds similar to those of sodium and potassium, although its carbonate and hydroxide were less soluble in water and more alkaline than the other alkali metals. Berzelius gave the unknown material the name "lithium /litina", from the Greek word λιθoς (transliterated as lithos, meaning "stone"), to reflect his discovery in a solid mineral, as opposed to potassium, which had been discovered in the ashes of plants. and sodium, which was known in part for its high abundance in animal blood. He named the metal within the material "lithium". Lithium, sodium, and potassium were part of the discovery of periodicity, being among a number of triads of elements in the same group that Johann Wolfgang Döbereiner noted in 1850 as having similar properties.
Rubidium and cesium were the first elements to be discovered using the spectroscope, invented in 1859 by Robert Bunsen and Gustav Kirchhoff. The following year, they discovered cesium in the mineral water of Bad Dürkheim, Germany. His discovery of rubidium occurred the following year in Heidelberg, Germany, and he found it in the mineral lepidolite. The names of rubidium and cesium come from the most prominent lines in their emission spectra: a bright red line for rubidium (from the Latin word rubidus, meaning dark red or bright red), and an azure blue line for cesium (derived from the Latin word caesius, meaning azure blue).
Around 1865, John Newlands produced a series of papers where he listed the elements in order of increasing atomic weight and similar physical and chemical properties repeating at intervals of eight; he compared that periodicity to octaves in music, where notes an octave apart have similar musical functions. His version brought together all the known alkali metals (lithium to cesium), as well as copper, silver, and thallium (showing the state oxidation characteristic +1 of alkali metals), in a group. His table placed hydrogen with the halogens.
After 1869, Dmitri Mendeleev proposed his periodic table by placing lithium at the top of a group with sodium, potassium, rubidium, cesium, and thallium. Two years later, Mendeleev revised his table, placing hydrogen in group 1 on lithium, and also moving thallium to the boron group. In this 1871 version, copper, silver, and gold were placed twice, once as part of group IB, and once as part of a "group VIII" encompassing current groups 8 to 11. After the introduction of the 18-column table, group IB elements were moved to their current position in the d block, while the alkali metals were left in the AI group. Later, the name of the group was changed to group 1 in 1988. The trivial name "alkali metals" it comes from the fact that the hydroxides of the group 1 elements are all strong alkalis when dissolved in water.
There were at least four wrong and incomplete discoveries before Marguerite Perey of the Institut Curie in Paris, France discovered francium in 1939 by purifying a sample of actinium-227, which had been reported to have an energy decay of 220 keV. However, Perey noted decay particles with an energy level below 80 keV. Perey thought that this decay activity might have been caused by a previously unidentified decay product, one that was separated during purification but arose again from pure actinium -227. Various tests ruled out the possibility that the unknown element is thorium, radium, lead, bismuth, or thallium. The new product exhibited chemical properties of an alkali metal (such as coprecipitating with cesium salts), leading Perey to believe it was element 87, caused by the alpha decay of actinium-227. Perey then attempted to determine the ratio of beta decay to alpha decay in actinium-227. Her first test put the alpha branch at 0.6%, a figure she later revised to 1%.
The next element below francium (eka-francium) on the periodic table would be ununenium (Uue), element 119. The synthesis of ununenium was first attempted in 1985 by bombarding an einsteinium -254 target with calcium ions -48 at the superHILAC accelerator in Berkeley, California. No atoms were identified, leading to a limiting yield of 300 nb.
It is highly unlikely that this reaction could create ununenium atoms in the near future, given the extremely difficult task of producing sufficient quantities of einsteinium-254, which is favored for the production of ultra-heavy elements due to its large size. mass, relatively long half-life of 270 days, and availability in significant quantities of several micrograms, to make a target large enough to increase the sensitivity of the experiment to the required level; Einsteinium has not been found in nature and has only been produced in laboratories, and in smaller amounts than are needed for the effective synthesis of superheavy elements. However, since ununenium is only the first element of period 8 in the extended periodic table, it may well be discovered in the near future through other reactions, and indeed an attempt to synthesize it is currently underway in Japan., none of the period 8 elements have yet been discovered, and it is also possible, due to trickle instability, that only lower period 8 elements, up to element 128, are physically possible. No attempts have been made of synthesis for heavier alkali metals: due to their extremely high atomic number, they would require new and more powerful methods and technology to make them.
Occurrence
In the Solar System

The Oddo-Harkins rule holds that elements with even atomic numbers are more common than those with odd atomic numbers, with the exception of hydrogen. This rule argues that elements with odd atomic numbers have one unpaired proton and are more likely to capture another, thus increasing their atomic number. In elements with even atomic numbers, the protons are paired, with each member of the pair compensating for the spin of the other, improving stability. All of the alkali metals have odd atomic numbers and are not as common as elements with even atomic numbers. adjacent gases (the noble gases and the alkaline earth metals) in the Solar System. The heavier alkali metals are also less abundant than the lighter ones, since alkali metals from rubidium onwards can only be synthesized in supernovae and not in stellar nucleosynthesis. Lithium is also much less abundant than sodium and potassium, as it is little synthesized in Big Bang nucleosynthesis and in stars: the Big Bang can only produce trace amounts of lithium, beryllium, and boron due to the absence of a nucleus. stable with 5 or 8 nucleons, and stellar nucleosynthesis could only bypass this bottleneck through the triple alpha process, fusing three helium nuclei to form carbon and jumping over those three elements.
On Earth
Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the formation and evolution of the solar system. In turn, Earth's natural history caused parts of this planet to have different concentrations of the elements. The mass of the Earth is approximately 5.98 ×1024 kg. It consists mainly of iron (32.1%), oxygen (30.1%), silicon (15.1%), magnesium (13.9%), sulfur (2.9%), nickel (1.8%), calcium (1.5%) and aluminum (1, 4%); The remaining 1.2% consists of small amounts of other elements. Due to planetary differentiation, the central region is believed to be composed primarily of iron (88.8%), with small amounts of nickel (5.8%), sulfur (4.5%), and less than 1% trace elements.
Alkali metals, due to their high reactivity, do not occur naturally in their pure state. They are lithophilic and therefore remain close to the Earth's surface because they readily combine with oxygen and thus strongly associate with silica, forming relatively low-density minerals that do not sink to the core of the earth. Potassium, rubidium, and cesium are also incompatible elements due to their large ionic radii.
Sodium and potassium are both very abundant on earth, both of which are among the ten most common elements in the earth's crust; sodium makes up about 2.6% of the weight of the earth's crust, making it the sixth most abundant element and the most abundant alkali metal. Potassium makes up about 1.5% of the earth's crust and is the seventh most abundant element. Sodium is found in many different minerals, the most common of which is ordinary salt (sodium chloride), which occurs in large amounts dissolved in seawater. Other solid deposits include halite, amphibole, cryolite, nitratin, and zeolite. Many of these solid deposits occur as a result of evaporation from ancient seas, which still occur now in places like Utah's Great Salt Lake and the Dead Sea. Despite its nearly equal abundance in the Earth's crust, sodium is much more common than potassium in the ocean, both because potassium's larger size makes its salts less soluble, and because potassium is bound by silicates in the ocean. Soil and potassium leaches are much more easily absorbed by plant life than sodium.
Despite its chemical similarity, lithium is generally not produced together with sodium or potassium due to its smaller size. Due to its relatively low reactivity, it can be found in seawater in large quantities; its estimated concentration in seawater is about 0.14 to 0.25 parts per million (ppm) or 25 micromolar. Its diagonal relationship to magnesium often allows it to replace magnesium in ferromagnesium ores, where its concentration in the crust is about 18 ppm, comparable to that of gallium and niobium. Commercially, the most important lithium mineral is spodumene, which is found in large deposits around the world.
Rubidium is about as abundant as zinc and more abundant than copper. It occurs naturally in the minerals leucite, pollucite, carnallite, zinnwaldite, and lepidolite, although none of these contain solely rubidium and there are no other alkali metals. Cesium is more abundant than some commonly known elements, such as antimony, cadmium, tin, and tungsten, but it is much less abundant than rubidium.
Francium-223, the only naturally occurring isotope of francium, is the alpha decay product of actinium-227 and can be found in trace amounts in uranium ores. In a given sample of uranium, it is it is estimated that there is only one francium atom for every 1018 uranium atoms. 22 minute short.
Properties
Physics and chemistry
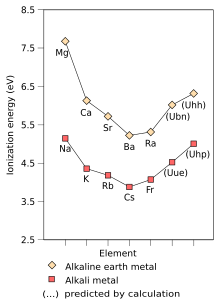
The physical and chemical properties of alkali metals can be easily explained by having a ns1 valence electron configuration, resulting in a weak metallic bond. Therefore, all alkali metals are soft and have low densities, melting, and boiling points, as well as sublimation, vaporization, and dissociation temperatures. They all crystallize in the body-centered cubic crystal structure, and have distinctive flame colors because their outer electron is very easily excited. The ns1 configuration also results in the alkali metals having very large atomic and ionic radii, as well as very high thermal and electrical conductivity. Its chemistry is dominated by the loss of its lone valence electron in the outermost s-orbital to form the +1 oxidation state, due to the ease of ionization of this electron and the very high second ionization energy. Most of the chemistry has been observed only for the first five members of the group. The chemistry of francium is not well established due to its extreme radioactivity; therefore, the presentation of its properties here is limited. The little that is known about francium shows that it behaves very close to cesium, as expected.
| Name | Lithium | Sodium | Potassium | Blonde | Cesio | Francio |
|---|---|---|---|---|---|---|
| Atomic | 3 | 11 | 19 | 37 | 55 | 87 |
| Atomic weight (u) | 6.94(1) | 22.98976928(2) | 39.0983(1) | 85.4678(3) | 132.9054519(2) | [223] |
| Electronic configuration | [He] 2s1 | [Ne] 3s1 | [Ar] 4s1 | [Kr] 5s1 | [Xe] 6s1 | [Rn] 7s1 |
| Merge point (°C) | 180.54 | 97.72 | 63.38 | 39.31 | 28.44 | ? |
| Evaporation point (°C) | 1342 | 883 | 759 | 688 | 671 | ? |
| Density (g·cm−3) | 0.534 | 0.968 | 0.89 | 1.532 | 1.93 | ? |
| Entalpía de fusion (kJ·mol−1) | 3.00 | 2.60 | 2.321 | 2.19 | 2.09 | ? |
| Steaming detail (kJ·mol−1) | 136 | 97.42 | 79.1 | 69 | 66.1 | ? |
| Training of a monoatomic gas (kJ·mol)−1) | 162 | 108 | 89.6 | 82.0 | 78.2 | ? |
| Electrical conductivity at 25 °C (nΩ·cm) | 94.7 | 48.8 | 73.9 | 131 | 208 | ? |
| Atomic radio (pm) | 152 | 186 | 227 | 248 | 265 | ? |
| Ionic Radio of an ion M+ hexacoordinate (pm) | 76 | 102. | 138 | 152 | 167. | ? |
| First inonization energy (kJ·mol−1) | 520.2 | 495.8 | 418.8 | 403.0 | 375.7 | 392.8 |
| Electronic affinity (kJ·mol)−1) | 59.62 | 52.87 | 48.38 | 46.89 | 45.51 | ? |
| M dissociation detail2(kJ·mol)−1) | 106.5 | 73.6 | 57.3 | 45.6 | 44.77 | ? |
| Pauling Scale | 0.98 | 0.93 | 0.82 | 0.82 | 0.79 | ? |
| Normal electrode potential (E°(M+→M0(V) | −3.04 | −2.71 | −2.93 | −2.98 | -3.03 | ? |
| Flame color
emission wavelength / main absorption (nm) | Carmesi 670.8 | Yellow 589.2 | Violeta 766.5 | Red-violaceous 780.0 | Blue
455.5 | ? |
The alkali metals are more similar to each other than elements in any other group are to each other. In fact, the similarity is so great that it is quite difficult to separate potassium, rubidium, and cesium, due to their similar ionic radii; Lithium and sodium are more distinct. For example, moving down the table, all known alkali metals show an increase in atomic radius, decrease in electronegativity, increase in reactivity, and decrease in melting and boiling points, as well as heats. melting and vaporization. In general, their densities increase further down the group, with the exception that potassium is less dense than sodium. One of the few properties of the alkali metals that does not show a very smooth trend is their reduction potential.: the value of lithium is anomalous, being more negative than the others. This is because the Li ion + has a very high hydration energy in the gas phase: although the lithium ion alters This high hydration energy is sufficient to cause reduction potentials to indicate it as being the most electropositive alkali metal, despite the difficulty of ionizing it in the gas phase.
The stable alkali metals are all silver in color, except cesium, which has a pale gold tint: it is one of three metals that are clearly colored (the other two are copper and gold). In addition, the heavy alkaline earth metals calcium, strontium, and barium, as well as the divalent lanthanides europium and ytterbium, are pale yellow in color, although the color is much less prominent than that of cesium. Their luster dulls rapidly in air due to oxidation. They all feature a cubic crystal system, and have distinctive flame colors because their outer electron is very easily excited. In fact, these flame test colors are the most common way to identify them, since all of their salts with common ions are soluble.
All alkali metals are highly reactive and are never found in elemental forms in nature. Because of this, they are usually stored in mineral oil or kerosene (paraffin oil). They react aggressively with halogens to form halides. alkali metals, which are white ionic crystalline compounds that are all soluble in water except lithium fluoride (LiF). Alkali metals also react with water to form strongly alkaline hydroxides and therefore must be handled with great care. careful. The heavier alkali metals react more vigorously than the lighter ones; for example, when dropped into water, cesium produces a larger explosion than potassium if the same number of moles of each metal is used. The alkali metals have the lowest first ionization energies in their respective periods in the table periodic due to their low effective nuclear charge and the ability to achieve a noble gas configuration by losing only one electron. The alkali metals not only react with water, but also with proton donors such as alcohols and phenols, gaseous ammonia and alkynes, the latter demonstrating the phenomenal degree of their reactivity. Their great power as reducing agents makes them very useful for freeing other metals from their oxides or halides.
The second ionization energy of all alkali metals is very high since they are in a complete shell that is also closer to the nucleus; thus, they almost always lose a single electron, forming cations. Alkalis are an exception, they are unstable compounds containing alkali metals in a -1 oxidation state, which is very unusual since prior to the discovery of alkalis, alkali metals were not expected to be able to form anions and were thought to be capable of appearing in salts only as cations. Alkaline anions have filled s-subshells, which gives them enough stability to exist. It is known that all stable alkali metals except lithium can form alkalis, and the alkalis are of much theoretical interest due to their unusual stoichiometry and low ionization potentials. Alkalides are chemically similar to electrides, which are salts with trapped electrons that act as anions. A particularly striking example of an alkali is the "inverse sodium hydride", H+Na- (both ions complexed), unlike the usual sodium hydride, Na+H- is unstable in isolation, due to to its high energy resulting from the shift of two electrons from hydrogen to sodium, although several derivatives are predicted to be metastable or stable.
In aqueous solution, alkali metal ions form aqua ions of the formula [M (H2O)n]+, where n is the solvation number. Their coordination numbers and shapes agree well with those expected from their ionic radii. In aqueous solution, water molecules directly attached to the metal ion are said to belong to the first coordination sphere, also known as the first, or primary, solvation shell. The bond between a water molecule and the metal ion is a dative covalent bond, with the oxygen atom donating both electrons to the bond. Each coordinated water molecule can be hydrogen bonded to other water molecules. The latter are said to reside in the second coordination sphere. However, for alkali metal cations, the second coordination sphere is not well defined as the +1 charge on the cation is not high enough to polarize the water molecules in the primary solvation shell enough to they form strong hydrogen bonds with those in the second coordination sphere, producing a more stable entity. The solvation number for Li + has been determined experimentally to be 4, forming the tetrahedral [Li(H2O)4]+; while solvation numbers of 3 to 6 have been found for lithium ions, solvation numbers less than 4 may result from contact ion pair formation and higher solvation numbers may be interpreted in terms of molecules of water that approximate [Li (H2O)4]+ through one face of the tetrahedron, although molecular dynamics simulations may indicate the existence of an octahedral hexaaqua ion. There are also probably six water molecules in the primary solvation sphere of the sodium ion, forming the octahedral ion [Na(H2O)6]+. While it was previously thought that the heavier alkali metals also form octahedral hexaaqua ions, it has since been discovered that potassium and rubidium probably form the ions [K(H2O)8]+ and [Rb (H2O)8]+, which have the square antiprismatic structure, and that cesium forms the 12-coordinate ion [Cs(H2O)12]+.
Lithium
The chemistry of lithium shows several differences from the rest of the group, as the small cation Li + polarizes the anions and gives its compounds a more covalent character. Lithium and lithium Magnesium are diagonally related due to their similar atomic radii, so they show some similarities. For example, lithium forms a stable nitride, a property common among all alkaline earth metals (magnesium group) but unique among the alkali metals. Also, among their respective groups, only lithium and magnesium form organometallic compounds with a character significant covalent
Lithium fluoride is the only alkali metal halide that is sparingly soluble in water, and lithium hydroxide is the only alkali metal hydroxide that is not deliquescent. By contrast, lithium perchlorate and other Lithium salts with large anions that cannot be polarized are much more stable than the analogous compounds of the other alkali metals, probably because Li + has a high solvation energy. This effect also means that most simple lithium salts are commonly found in the hydrated form, because the anhydrous forms are extremely hygroscopic, this allows salts such as lithium chloride and lithium bromide to be used in dehumidifiers and air conditioners..
France
Francium is predicted to show some differences due to its high atomic weight, causing its electrons to travel at considerable fractions of the speed of light and thus making relativistic effects more prominent. In contrast to the decreasing trend of the electronegativities and ionization energies of the alkali metals, francium's electronegativity and ionization energy are predicted to be higher than those of cesium due to relativistic stabilization of the 7s electrons; Furthermore, its atomic radius is expected to be abnormally low. Therefore, contrary to expectation, cesium is the most reactive of the alkali metals, not francium. All known physical properties of francium also deviate from the clear trends for lithium to cesium, such as first ionization energy, electron affinity, and anion polarization, although due to the paucity of known data on francium, many sources give extrapolated values, ignoring that relativistic effects make the trend from lithium to cesium inapplicable in the Some of the few properties of francium that have been predicted taking relativity into account are the electron affinity (47.2 kJ/mol) and the enthalpy of dissociation of the molecule Fr 2 (42.1 kJ / mol). The CsFr molecule is polarized as Cs+Fr-, showing that the francium 7s subshell is much more affected by relativistic effects than the francium 7s subshell. cesium 6s subshell. In addition, it is expected that the super Francium roxide (FrO2) has significant covalent character, unlike the other alkali metal superoxides, due to the bonding contributions of francium's 6p electrons.
Nuclear
| Z | Alkaline metal | Stable | Decade | unstable: italics | ||
|---|---|---|---|---|---|---|
| 3 | Lithium | 2 | - | 7Li | 6Li | |
| 11 | sodium | 1 | - | 23Na | ||
| 19 | potassium | 2 | 1 | 39K | 41K | 40K |
| 37 | ruby | 1 | 1 | 85Rb | 87Rb | |
| 55 | cesio | 1 | - | 133Cs | ||
| 87 | French | - | - | There are no primordial isotopes (223Fr is a radiogenic nucleide) | ||
| Radioactive: 40K, t1/2 1.25 × 109 years; 87Rb, t1/2 4.9 × 1010 years; 223Fr, t1/2 22.0 min. | ||||||
All the alkali metals have odd atomic numbers; therefore, their isotopes must be either odd-odd (both the number of protons and neutrons are odd) or odd-even (the number of protons is odd, but the number of neutrons is even). Odd nuclei have even mass numbers, while odd nuclei have odd mass numbers. Odd and odd primordial nuclides are rare because most odd and odd nuclei are very unstable with respect to beta decay, because the decay products are even and even, and therefore more tightly bound, due to the effects of nuclear pairing.
Due to the great rarity of odd and odd nuclei, nearly all of the primordial isotopes of the alkali metals are odd and even (with the exception of the light-stable isotope lithium-6 and the long-lived radioisotope potassium-40). For a given odd mass number, there can only be a single stable beta nuclide, since there is no difference in binding energy between even-odd and odd, even comparable to even-even and odd-odd, leaving others nuclides of the same mass number (isobars) free beta decay towards the nuclide of lower mass. One effect of the instability of an odd number of either type of nucleon is that odd-numbered elements, such as the alkali metals, tend to have fewer stable isotopes than even-numbered elements. Of the 26 monoisotopic elements that have only one stable isotope, all but one have an odd atomic number, and all but one also have an even number of neutrons. Beryllium is the only exception to both rules, due to its low atomic number.
All alkali metals except lithium and cesium have at least one naturally occurring radioisotope: sodium-22 and sodium-24 are cosmogenically produced trace radioisotopes, potassium-40 and rubidium-87 have very long half-lives and, therefore, occur naturally, and all francium isotopes are radioactive. Cesium was also thought to be radioactive in the early 20th century, although it has no naturally occurring radioisotopes. (Francium had not yet been discovered at the time.) The natural long-lived radioisotope of potassium, potassium-40, makes up about 0.012% of natural potassium, and therefore natural potassium is weakly radioactive. This naturally occurring radioactivity became a basis for an erroneous claim for the discovery of element 87 (the next alkali metal after cesium) in 1925. Natural rubidium is similarly slightly radioactive, with 27.83% being the long-lived radioisotope rubidium. -87.
Cesium-137, with a half-life of 30.17 years, is one of the two main half-life fission products, along with strontium-90, that are responsible for most of the radioactivity in spent nuclear fuel after several years of cooling, up to several hundred years after use. It makes up most of the radioactivity still left from the Chernobyl accident. Cesium-137 undergoes high-energy beta decay and eventually becomes stable barium-137. It is a strong emitter of gamma radiation. Cesium-137 has a very low rate of neutron capture and cannot be removed in this way, but must be allowed to decay. Cesium-137 has been used as a tracer in hydrological studies, analogous to the use of tritium. Small amounts of cesium-134 and cesium-137 were released into the environment during almost all nuclear weapons tests and some nuclear accidents, notably the Goiânia accident and the Chernobyl disaster. As of 2005, cesium-137 is the main source of radiation in the zone of alienation around the Chernobyl nuclear power plant. Its chemical properties as one of the alkali metals make it one of the most problematic fission products of short to half-life because it moves and spreads easily in nature due to the high water solubility of its salts, and is absorbed by the body, which confuses it with its essential congeners sodium and potassium.
Periodic Trends
The alkali metals are more similar to each other than elements in any other group. For example, moving down the table, all known alkali metals show an increase in atomic radius, decrease in electronegativity, increased reactivity, and decreased melting and boiling points, as well as heats of fusion and vaporization. In general, their densities increase as they come down from the table, with the exception that potassium is less dense than sodium.
Atomic and ionic radii
The atomic radii of alkali metals increase down the group. Due to the shielding effect, when an atom has more than one electron shell, each electron feels electrical repulsion from the other electrons, as well as electrical attraction from the nucleus. In alkali metals, the outermost electron only feels a net charge of +1, since part of the nuclear charge (which is equal to the atomic number) is canceled by the inner electrons; The number of inner electrons of an alkali metal is always one less than the nuclear charge. Therefore, the only factor that affects the atomic radius of alkali metals is the number of electron shells. As this number increases over the group, the atomic radius must also increase over the group.
The ionic radii of alkali metals are much smaller than their atomic radii. This is because the outermost electron of the alkali metals is in a different electron shell than the inner electrons, and therefore, when it is removed, the resulting atom has one fewer electron shell and is smaller. Also, the effective nuclear charge has increased and therefore electrons are more strongly attracted towards the nucleus and the ionic radius decreases.
First Ionization Energy
The first ionization energy of an element or molecule is the energy required to move the loosest electron in one mole of gaseous atoms of the element or molecules to form one mole of gaseous ions with +1 electrical charge. Factors that affect the first ionization energy are the nuclear charge, the amount of shielding of the inner electrons, and the distance of the most weakly held electron from the nucleus, which is always an outer electron in main group elements. The first two factors change the effective nuclear charge felt by the looser electron. Since the outermost electron of alkali metals always feels the same effective nuclear charge (+1), the only factor affecting the first ionization energy is the distance from the outermost electron to the nucleus. As this distance increases in the group, the outermost electron feels less attraction from the nucleus and therefore the first ionization energy decreases. (This trend is broken in francium due to relativistic stabilization and contraction of the 7s orbital, which brings the valence electron of francium closer to the nucleus than would be expected from non-relativistic calculations. This makes the valence electron of francium The outermost francium feels more of a pull from the nucleus, increasing its first ionization energy slightly beyond that of cesium).
The second ionization energy of alkali metals is much higher than the first, since the second loosest electron is part of a completely filled electron shell and therefore difficult to remove.
Reactivity
The reactivities of the alkali metals increase going down the group. This is the result of a combination of two factors: the first ionization energies and the atomization energies of the alkali metals. Because the first ionization energy of alkali metals decreases in the group, it is easier for the outermost electron to be removed from the atom and participate in chemical reactions, thus increasing the reactivity in the group. Atomization energy measures the strength of an element's metallic bond, which falls in the group as the atoms increase in radius and therefore the metallic bond must increase in length, causing the delocalized electrons to move further apart than the attraction of the nuclei of the heavier nuclei of the alkali metals. Adding the energies of atomization and first ionization gives a quantity closely related to (but not equal to) the activation energy of the reaction of an alkali metal with another substance. This quantity decreases going down the group, and so does the activation energy; therefore, chemical reactions can occur faster and reactivity increases in the group.
Electronegativity
Electronegativity is a chemical property that describes the tendency of an atom or functional group to attract electrons (or electron density) toward itself. If the bond between sodium and chlorine in sodium chloride were covalent, the shared pair of electrons would be attracted to chlorine because the effective nuclear charge on the outer electrons is +7 in chlorine but is only +1 in sodium. The pair of electrons is attracted so close to the chlorine atom that they are practically transferred to the chlorine atom (an ionic bond). However, if the sodium atom was replaced by a lithium atom, the electrons will not be attracted as close to the chlorine atom as before because the lithium atom is smaller, making the pair of electrons more strongly attracted to the nearest effective nuclear charge of lithium. Therefore, larger alkali metal atoms (lower down in the group) will be less electronegative since the bonding pair is less attracted to them. Francium is expected to be an exception.
Due to the higher electronegativity of lithium, some of its compounds have a more covalent character. For example, lithium iodide (Li I) will dissolve in organic solvents, a property of most covalent compounds. Lithium fluoride (Li F) is the only alkali halide that is not soluble in water, and lithium hydroxide (LiOH) is the only alkali metal hydroxide that is not deliquescent.
Melting and boiling points
The melting point of a substance is the point where it changes from a solid to a liquid state, while the boiling point of a substance (in a liquid state) is the point where the vapor pressure of the liquid equals the pressure environment surrounding the liquid and the entire liquid changes state to a gas. When a metal is heated to its melting point, the metallic bonds that hold the atoms in place are weakened so that the atoms can move, and the metallic bonds eventually break completely at the boiling point of the metal. Thus, the falling melting and boiling points of the alkali metals indicates that the strength of the metal bonds of the alkali metals decreases in the group. This is because the metal atoms are held together by electromagnetic attraction. from the positive ions to the delocalized electrons. As the atoms increase in size down the group (because their atomic radius increases), the nuclei of the ions move further away from the delocalized electrons and thus, the metallic bond is weakened so that the metal melts and boils more easily, thus lowering the melting and boiling points. (The increase in nuclear charge is not a relevant factor due to the d effect and armor).
Density
All alkali metals have the same crystal structure (body-centered cubic), and therefore the only relevant factors are the number of atoms that can fit in a given volume and the mass of one of the atoms, since density is defined as mass per unit volume. The first factor depends on the volume of the atom and, therefore, on the atomic radius, which increases as the group descends; thus the volume of an alkali metal atom increases down the group. The mass of an alkali metal atom also increases down the group. Therefore, the trend of the densities of the alkali metals depends on their atomic weights and atomic radii; If the figures for these two factors are known, the relationships between the densities of the alkali metals can be calculated. The resulting trend is for the densities of the alkali metals to increase in the table, with the exception of potassium. Because they have the lowest atomic weight and largest atomic radius of all the elements in their periods, the alkali metals are the least dense metals on the periodic table. Lithium, sodium, and potassium are the only three metals on the periodic table they are less dense than water: in fact, lithium is the least dense solid known at room temperature.
Compounds
The alkali metals form a complete series of compounds with all the anions usually found, which well illustrate the tendencies of the group. These compounds can be described as involving the alkali metals that lose electrons to acceptor species and form monopositive ions. This description is more accurate for alkali halides and becomes less and less accurate as the cationic and anionic charge increases, and as the anion becomes larger and more polarizable. For example, ionic bonding gives way to metallic bonding along the series NaCl, Na2O, Na2S, Na3 >P, Na3As, Na3Sb, Na3Bi
Hydroxides
All alkali metals react vigorously or explosively with cold water, producing an aqueous solution of a strongly basic alkali metal hydroxide and liberating hydrogen gas. This reaction becomes more vigorous further down the group, lithium constantly reacts with effervescence, but sodium and potassium can ignite and rubidium and cesium sink in water and generate hydrogen gas so rapidly that shock waves are formed in the water that can break glass containers. When an alkali metal is dropped into water, it produces an explosion, of which there are two separate stages. The metal first reacts with water, breaking the hydrogen bonds in the water and producing hydrogen gas; this occurs faster for the heavier, more reactive alkali metals. Second, the heat generated by the first part of the reaction often ignites hydrogen gas, causing it to burn explosively in the surrounding air. This secondary explosion of hydrogen gas produces the visible flame over the bowl of water, lake, or other body of water, not the initial reaction of metal with water (which tends to occur primarily underwater). Hydroxides of alkali metals are the most basic hydroxides known.
Recent research has suggested that the explosive behavior of alkali metals in water is driven by a Coulomb explosion and not just by the rapid generation of hydrogen. All alkali metals melt as part of the reaction with water. The water molecules ionize the bare metal surface from the liquid metal, leaving a positively charged metal surface and negatively charged water ions. The attraction between the charged metal and the water ions will rapidly increase the surface area, causing an exponential increase in ionization. When the repulsive forces within the surface of the liquid metal exceed the forces of surface tension, it explodes vigorously.
The hydroxides themselves are the most basic hydroxides known, they react with acids to give salts and with alcohols to give oligomeric alkoxides. They readily react with carbon dioxide to form carbonates or bicarbonates, or with hydrogen sulfide to form sulfides or disulfides, and can be used to separate thiols from petroleum. They react with amphoteric oxides: for example, oxides of aluminum, zinc, tin, and lead react with alkali metal hydroxides to give aluminates, zincates, stannates, and plumbers. Silicon dioxide is acidic and therefore alkali metal hydroxides can also attack silicate glass.
Intermetallic compounds
The alkali metals form many intermetallic compounds with each other and elements in groups 2 to 13 on the periodic table of variable stoichiometries, such as sodium-mercury amalgams, including Na5Hg8 and Na3Hg. Some of these have ionic characteristics: take alloys with gold, the most electronegative metal, for example, NaAu and KAu are metallic, but RbAu and CsAu are semiconductors. NaK is an alloy of sodium and potassium that is very useful because it is liquid at room temperature, although precautions must be taken due to its extreme reactivity towards water and air. The eutectic mixture melts at −12.6 °C. An alloy of 41% cesium, 47% sodium, and 12% potassium has the lowest known melting point of any metal or alloy, −78 °C.
Compounds with the elements of group 13
Intermetallic compounds of alkali metals with the heavier group 13 elements (aluminum, gallium, indium, and thallium), such as NaTl, are poor conductors or semiconductors, unlike normal alloys with the above elements, which implies that the alkali metal involved has lost an electron to the Zintl anions involved. However, while elements in group 14 and beyond tend to form discrete anionic groups, group 13 elements tend to form polymer ions with the cations. of alkali metals located between the giant ionic lattice. For example, NaTl consists of a polymeric anion (—Tl - -)n with a cubic covalent diamond structure with Na + ions located between the anionic lattice. The larger alkali metals cannot similarly fit into an anionic lattice and tend to force the heavier group 13 elements into anionic groups.
Boron is a special case, as it is the only nonmetal in group 13. The alkali metal borides tend to be boron-rich, implying appreciable boron-boron bonding involving deltahedral structures, and they are thermally unstable because the alkali metals have a very high vapor pressure at elevated temperatures. This makes direct synthesis problematic because the alkali metals do not react with boron below 700 °C, and therefore this must be accomplished in sealed containers with the excess alkali metal. Also, exceptionally in this group, the reactivity with boron decreases in the group: lithium fully reacts at 700 °C, but sodium at 900 °C and potassium not until 1200 °C, and the reaction is instantaneous for lithium, but takes hours for potassium. Rubidium and cesium borides have not even been characterized. Several phases are known, such as LiB10, NaB6, NaB15, and KB6. Low High pressure, the boron-boron bonding in lithium borides changes from following Wade's rules to forming Zintl anions like the rest of group 13.
Compounds with the elements of group 14
Lithium and sodium react with carbon to form acetylides, Li2C2 and Na2C2, which can also be obtained by reacting metal with acetylene. Potassium, rubidium, and cesium react with graphite; their atoms intercalate between the layers of hexagonal graphite, forming graphite intercalation compounds of the formulas MC60 (dark gray, almost black), MC48 (dark gray, almost black), MC36 (blue), MC24 (steel blue) and MC8 (bronze) (M = K, Rb or Cs). These compounds are more than 200 times more electrically conductive than pure graphite, suggesting that the valence electron from the alkali metal is transferred to the graphite layers (eg M+
/span>C−
8). By heating KC8, the removal of carbon atoms potassium results in sequential conversion to KC24, KC36, KC48 and finally KC60. KC8 is a very strong reducing agent and is pyrophoric and explodes on contact with water. While the larger alkali metals (K, Rb and Cs) initially form MC8, the smaller ones initially form MC6, and in fact require the reaction of the metals with graphite at high temperatures around 500 °C to form. Other than this, the alkali metals are reducing agents so strong that they can even reduce buckminsterfullerene to produce solid MnC60 fullerides; sodium, potassium, rubidium and cesium can form fullerides where n = 2, 3, 4 or 6, and rubidium and cesium can additionally reach n = 1.
When alkali metals react with the heavier elements in the carbon group (silicon, germanium, tin and lead), ionic substances with cage-like structures are formed, such as M4Si4 (M = K, Rb, or Cs), containing M+ and tetrahedral ions Yes4−
4. The chemistry of the alkali metal germanides, involving the germanide ion Ge4− and other cluster ions (Zintl) as Ge2−
4, Ge4−
9, Ge2−
9, and [(Ge9)2]6 −, is largely analogous to that of the corresponding silicides.Alkali metal stannides are mostly ionic, sometimes with the stannide ion (Sn4−), and sometimes with more complex Zintl ions like Sn4−
9, which occurs in tetrapotassium nonastanide (K4Sn9). The monatomic ion of the plumb bob (Pb4−) is unknown, and indeed its formation is predicted to be energetically unfavorable, alkali metal plasmids have complex Zintl ions, such as Pb4−
9. These alkali metal germanides, stannides, and plasmids can be produced by reducing germanium, tin, and lead with metallic sodium in liquid ammonia.
Nitrides and pnictids
Lithium, the lightest of the alkali metals, is the only alkali metal that reacts with nitrogen under standard conditions, and its nitride is the only stable alkali metal nitride. Nitrogen is an unreactive gas because breaking the strong triple bond in the dinitrogen molecule (N2) requires a lot of energy. The formation of an alkali metal nitride would consume the ionization energy of the alkali metal (forming M+ ions), the energy required to break the triple bond at N2, and the formation of N3− ions, and all the energy released from the formation of an alkali metal nitride comes from the lattice energy of the alkali metal nitride. The lattice energy is maximized with small, highly charged ions; alkali metals do not form highly charged ions, they only form ions with a charge of +1, so only lithium, the smallest alkali metal, can release enough lattice energy to make the reaction with nitrogen exothermic, forming lithium nitride. lithium. Reactions of the other alkali metals with nitrogen would not release enough lattice energy and would therefore be endothermic, so they do not form nitrides under standard conditions. Sodium nitride (Na3N) and potassium nitride (K3N), although they exist, are extremely unstable, prone to decompose back into their constituent elements, and cannot be produced by reacting the elements with each other under standard conditions. Steric hindrance prohibits the existence of rubidium or cesium nitride. However, sodium and potassium form colorless azide salts involving the anion N−
3 linear; that due to the large size of the alkali metal cations, they are thermally stable enough to be able to melt before decomposing.
All alkali metals readily react with phosphorus and arsenic to form phosphides and arsenides with the formula M3Pn (where M represents an alkali metal and Pn represents a pycnogen: phosphorus, arsenic, antimony, or bismuth). This is due to the larger size of the P3- and As3- ions, so less lattice energy needs to be released for the salts to form. they are the only phosphides and arsenides of the alkali metals; for example, potassium has nine different known phosphides, with formulas K3P, K4P3, K5P4, KP, K4P6, K3P7 >, K3P11, KP10.3 and KP15. While most metals form arsenides, only the alkali and alkaline earth metals form mainly ionic arsenides. The structure of Na3As is complex with unusually short Na-Na distances of 328-330 pm, which are shorter than in sodium metal, and this indicates that even with these electropositive metals, the union cannot be directly ionic. Other alkali metal arsenides that do not fit the formula M3As are known, such as LiAs, which has a metallic luster and electrical conductivity indicating the presence of some metallic bond. Antimonides are unstable and reactive since the Sb3 ion is a strong reducing agent; Reaction of these with acids forms the toxic and unstable gaseous stibnite (SbH3). In fact, they have some metallic properties, and the alkali metal antimonides of MSb stoichiometry involve bound antimony atoms in a Zintl spiral structure. Bismutids are not even fully ionic; are intermetallic compounds that contain partly metallic and partly ionic bonds.
Oxides
All alkali metals react vigorously with oxygen under standard conditions. They form various types of oxides, such as simple oxides (containing the O2− ion), peroxides (containing the O2−
2, where there is a single bond between the two oxygen atoms), superoxides (containing the ion O−
2), and many others. Lithium burns in air to form lithium oxide, but sodium reacts with oxygen to form a mixture of sodium oxide and sodium peroxide. Potassium forms a mixture of potassium peroxide and potassium superoxide, while rubidium and cesium form superoxides exclusively. Their reactivity increases down the group: while lithium, sodium, and potassium simply burn in air, rubidium and cesium are pyrophoric (ignite spontaneously in air).
Smaller alkali metals tend to polarize larger anions (peroxide and superoxide) due to their small size. This draws the electrons in the more complex anions toward one of their constituent oxygen atoms, forming an oxide ion and an oxygen atom. This causes lithium to form an oxide exclusively in reaction with oxygen at room temperature. This effect becomes drastically weaker for the larger sodium and potassium, allowing them to form the less stable peroxides. Rubidium and cesium, at the bottom of the group, are so large that even the least stable superoxides can form. Because superoxide releases the most energy when it forms, superoxide forms preferentially for the larger alkali metals where the more complex anions are not polarized. (Oxides and peroxides for these alkali metals exist, but are not formed by direct reaction of the metal with oxygen under standard conditions.) In addition, the small size of the Li+ and O ions 2− helps them form a stable ionic lattice structure. However, under controlled conditions, all the alkali metals, with the exception of francium, are known to form their oxides, peroxides, and superoxides. Alkali metal peroxides and superoxides are powerful oxidizing agents. Sodium peroxide and potassium superoxide react with carbon dioxide to form alkali metal carbonate and oxygen gas, allowing them to be used in submarine air purifiers; The presence of water vapor, naturally present in the breath, makes the removal of carbon dioxide by potassium superoxide even more efficient. All stable alkali metals, except lithium, can form red ozonides (MO3) through low temperature reaction of powdered anhydrous hydroxide with ozone: ozonides can be extracted with liquid ammonia.
Rubidium and cesium can form a wide variety of suboxides with metals in formal oxidation states below +1. Rubidium can form Rb6O and Rb9O2 (copper color) upon oxidation in air, while cesium forms a huge variety of oxides, such as ozonide OSC3 and various brightly colored suboxides, such as Cs7O (bronze), Cs4O (red-violet), Cs11O3 (violet), Cs3O (dark green), CsO, Cs 3O2, also as Cs7O2. The latter of these can be heated in a vacuum to generate Cs2O.
Halides, hydrides
The alkali metals are among the most electropositive elements on the periodic table, and therefore tend to bond ionically with the most electronegative elements on the periodic table, the halogens (fluorine, chlorine, bromine, iodine, and astatin)., forming salts known as alkali metal halides. The reaction is very energetic and can sometimes cause explosions. Twenty stable alkali metal halides are known; The unstable ones are unknown, with the exception of sodium astatate, due to the great instability and rarity of astatine and francium. The best known of the twenty is undoubtedly sodium chloride, also known as common salt. All stable alkali metal halides have the formula MX (where M is an alkali metal and X is a halogen). They are all white ionic crystalline solids that have high melting points. All of the alkali metal halides are soluble in water, except lithium fluoride (LiF), which is insoluble in water due to its high lattice enthalpy. The high lattice enthalpy of lithium fluoride is due to the small sizes of the Li+ and F- ions, which makes the electrostatic interactions between them strong; a similar effect occurs for magnesium fluoride, consistent with the diagonal relationship between lithium and magnesium.
Coordination complexes
Cations of alkali metals generally do not form coordination complexes with simple Lewis bases due to their low charge of only +1 and their relatively large size; thus, the Li+ ion forms most complexes, and the heavier alkali metal ions form to a lesser extent (although exceptions occur for weak complexes). Lithium in particular has a very rich coordination chemistry in which it exhibits coordination numbers from 1 to 12, although octahedral hexacoordination is its preferred mode. In aqueous solution, alkali metal ions exist as octahedral hexahydrate complexes [M(H2O)6)]+, with the exception lithium ion, which due to its small size forms tetrahedral tetrahydrate complexes [Li(H2O)4)]+; the alkali metals form these complexes because their ions are attracted by the electrostatic forces of attraction toward polar water molecules. Because of this, anhydrous salts containing alkali metal cations are often used as desiccants. The alkali metals also easily complex with crown ethers.
Ammonia solutions
Alkali metals dissolve slowly in liquid ammonia, forming ammoniacal solutions of metal cation solvate M+ and electron solvate e -, which react to form hydrogen gas and the alkali metal amide (MNH2, where M represents an alkali metal). The process can be accelerated by a catalyst. Similar solutions are formed by alkaline earth metals such as calcium, strontium, barium, as well as the divalent lanthanides, europium and ytterbium. The amide salt is quite insoluble and readily precipitates out of solution, leaving intensely colored ammonia solutions of the alkali metals. At low concentrations (below 3 M), the solution is dark blue and has ten times the conductivity of aqueous sodium chloride; at higher concentrations (greater than 3 M), the solution is copper-colored and has approximately the conductivity of liquid metals such as mercury. In addition to the alkali metal amide salt and solvated electrons, such ammonia solutions also contain the alkali metal cation (M+), the neutral alkali metal atom (M), diatomic alkali metal molecules (M2) and alkali metal anions (M-) These are unstable and eventually convert to the alkali metal amide and hydrogen more thermodynamically stable gas. Solvated electrons are powerful reducing agents and are often used in chemical synthesis.
Organometallic compounds
Organolithium
Being the smallest alkali metal, lithium forms the widest variety of most stable organometallic compounds, which are held together by covalent bonds. Organolithium compounds are volatile, electrically non-conductive solids or liquids that melt at low temperatures and tend to form oligomers with the structure (RLi)x where R is the group organic. Since the electropositive nature of lithium puts most of the bond's charge density on the carbon atom, effectively creating a carbanion, organolytic compounds are extremely powerful bases and nucleophiles. For use as bases, butyllithiums are often used and are commercially available. An example of an organolytic compound is methyl lithium ((CH3Li)x), which exists in tetrameric (x = 4, tetrahedral) and hexameric (x = 6, octahedral). Organolithium compounds, especially n- butyllithium, are useful reagents in organic synthesis, as might be expected given the diagonal relationship of lithium to magnesium, which plays an important role in the reaction of Grignard. For example, alkylithios and arylithios can be used to synthesize aldehydes and ketones by reaction with metal carbonyls. Reaction with nickel tetracarbonyl, for example, proceeds through an unstable acyl nickel carbonyl complex which then undergoes electrophilic substitution to give the desired aldehyde product (using H+ as the electrophile). or ketone (using an alkyl halide).
Li+[RCONi(CO)3]-}}}" xmlns="http://www.w3.org/1998/Math/MathML">LiR+[chuckles]Ni(CO)4]− − COΔ Δ Li+[chuckles]RCONi(CO)3]− − {displaystyle {ce {LiR + [Ni(CO)4]^{-CO}- Li+[RCONi(CO)3]-}}}}
Li+[RCONi(CO)3]-}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/4c9f7d7f9900b6c684d152c2b7e0e1ea4e5eeef1" style="vertical-align: -1.005ex; width:46.107ex; height:3.509ex;"/>
[{H+}][{solvente}] Li+ + RCHO + [(solvente)Ni(CO)3]}}}" xmlns="http://www.w3.org/1998/Math/MathML">Li+[chuckles]RCONi(CO)3]− − →sorlventeH+Li++RCHO+[chuckles](solvent)Ni(CO)3]{displaystyle {ce {Li+[RCONi(CO)3]- - wage[{H+}[{solvente}]] Li+ + RCHO + [(solvent)Ni(CO)3]}}}}}}}}
[{H+}][{solvente}] Li+ + RCHO + [(solvente)Ni(CO)3]}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/7a98a22736ba08102047e9ad327793dcba37eb7a" style="vertical-align: -1.812ex; margin-top: -0.329ex; margin-bottom: -0.526ex; width:65.222ex; height:5.676ex;"/>
[{R'Br}][{solvente}] Li+ + R'COR + [(solvente)Ni(CO)3]}}}" xmlns="http://www.w3.org/1998/Math/MathML">Li+[chuckles]RCONi(CO)3]− − →sorlventeR♫BrLi++R♫COR+[chuckles](solvent)Ni(CO)3]{displaystyle {ce {Li+[RCONi(CO)3]- - agraria[{R'Br}][{solvente}] Li+ + R'COR + [(solvente)Ni(CO)3]}}}}}}
[{R'Br}][{solvente}] Li+ + R'COR + [(solvente)Ni(CO)3]}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/9d105613b04b7666ec222d2d228b43a1e6979783" style="vertical-align: -1.812ex; margin-top: -0.429ex; margin-bottom: -0.526ex; width:65.874ex; height:6.009ex;"/>

Alkylthios and aryllithios can also react with disubstituted N, N- amides to give aldehydes and ketones, and symmetrical ketones when reacting with carbon monoxide. They decompose thermally to eliminate a β-hydrogen, producing alkenes and lithium hydride; another route is the reaction of ethers with alkyl and aryllithiums that act as strong bases. In nonpolar solvents, aryllithiums react as the carbanions that they are, effectively converting carbon dioxide to aromatic carboxylic acids (ArCO2 H) and aryl ketones to tertiary carbinols (Ar' 2C(Ar)OH). Finally, they can be used to synthesize other organometallic compounds through metal-halogen exchange.
Heavier alkali metals
Unlike organolytic compounds, organometallic compounds of the heavier alkali metals are predominantly ionic. The application of organosodium compounds in chemistry is limited in part due to competition from organolytic compounds, which are commercially available and exhibit more convenient reactivity. The main organosodium compound of commercial importance is sodium cyclopentadienide. Sodium tetraphenylborate can also be classified as an organosodium compound since in the solid state sodium is bound to aryl groups. Organometallic compounds of the higher alkali metals are even more reactive than organosodium compounds and of limited utility. One notable reagent is Schlosser's base, a mixture of n-butyllithium and potassium tert- butoxide. This reagent reacts with propene to form the compound allylpotassium (KCH2 CHCH2). cis -2-butene and trans -2-butene equilibrate when in contact with alkali metals. While isomerization is fast with lithium and sodium, it is slow with the heavier alkali metals. The heavier alkali metals also favor the sterically congested conformation. Various crystal structures of organopotassium compounds have been reported, establishing that they, like sodium compounds, are polymeric. Organosodium, organopotassium, organorubidium, and organocaesium compounds are mostly ionic and are insoluble (or nearly so) in nonpolar solvents.
Alkyl and aryl derivatives of sodium and potassium tend to react with air. They cause the cleavage of ethers, generating alkoxides. Unlike alkyl lithium compounds, sodium alkyls and potassium alkyls cannot be made by reacting the metals with alkyl halides because Wurtz coupling occurs
R - R' + MX}}}" xmlns="http://www.w3.org/1998/Math/MathML">RM+R♫XΔ Δ R− − R♫+MX{displaystyle {ce {RM + R'X-negative R-R' + MX}}}}
R - R' + MX}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/b1492d9f64670be6e1974052e2b15914385d6471" style="vertical-align: -0.505ex; width:28.545ex; height:2.676ex;"/>
As such, they must be made by reacting alkylmercury compounds with metallic sodium or potassium in inert hydrocarbon solvents. While methyl sodium forms tetramers like methyl lithium, methyl potassium is more ionic, having the nickel arsenide structure with discrete methyl anions and potassium cations.
Alkali metals and their hydrides react with acidic hydrocarbons, for example cyclopentadienes and terminal alkynes, to give salts. Liquid ammonia, ether, or hydrocarbon solvents are used, the most common of which is tetrahydrofuran. The most important of these compounds is sodium cyclopentadienide, NaC5 H5, an important precursor to many transition metal cyclopentadienyl derivatives. Similarly, alkali metals react with cyclooctatetraene in tetrahydrofuran to give alkali metal cyclooctatetraenides; for example, dipotassium cyclooctatetraenide (K2C8H8) is an important precursor to many metal derivatives of cyclooctatetraenyl, such as uranocene. Large, very weakly polarizing alkali metal cations can stabilize large, radically polarizable aromatic anions, such as the dark green sodium naphthalenide, Na+ [C10 H8•]-, a strong reducing agent.
Representative reactions of alkali metals
Reaction with oxygen
By reacting with oxygen, alkali metals form oxides, peroxides, superoxides, and suboxides. However, the first three are more common. The table below shows the types of compounds formed in reaction with oxygen. The compound in parentheses represents the minor product of combustion.
| Alkaline metal | Oxide | Peroxide | Superoxide |
| Li | Li2O | (Li2O2) | |
| Na | (Na2O) | Na2O2 | |
| K | KO2 | ||
| Rb | RbO2 | ||
| Cs | CsO2 |
Alkali metal peroxides are ionic compounds that are unstable in water. The peroxide anion weakly binds to the cation and hydrolyzes, forming stronger covalent bonds.
2 NAOH + H2O2}}}" xmlns="http://www.w3.org/1998/Math/MathML">Na2O+2H2OΔ Δ 2NAOH+H2O2{displaystyle {ce {Na2O + 2 H2O - 2005 2 NAOH + H2O2}}}}}
2 NAOH + H2O2}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/6e59f1cfaf855b6c33324b2560bfa7636f745078" style="vertical-align: -1.005ex; width:36.946ex; height:2.843ex;"/>
Other oxygen compounds are also unstable in water.
2 KOH + H2O2 +O2}}}" xmlns="http://www.w3.org/1998/Math/MathML">2KO2+2H2OΔ Δ 2KOH+H2O2+O2{displaystyle {ce {2 KO2 + 2 H2O) 2 KOH + H2O2 +O2}
2 KOH + H2O2 +O2}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/a5d6e014bd97a8b160259abd11ea47377b176dac" style="vertical-align: -1.005ex; width:41.423ex; height:2.843ex;"/> 2 LiOH}}}" xmlns="http://www.w3.org/1998/Math/MathML">Li2O+H2OΔ Δ 2LiOH{displaystyle {ce {Li2O + H2O - 2005 2 LiOH}}}}
2 LiOH}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/bb30e2511132e1eb9698f730a2b878e189a1941c" style="vertical-align: -1.005ex; width:24.704ex; height:2.843ex;"/>
Reaction with sulfur
With sulfur, they form sulfides and polysulfides.
Na2S + 1/8S8 -> Na2S2...Na2S7}}}" xmlns="http://www.w3.org/1998/Math/MathML">2Na+18S8Δ Δ Na2S+18S8Δ Δ Na2S2⋅ ⋅ ⋅ ⋅ ⋅ ⋅ Na2S7{displaystyle {ce {2Na + 1/8S8 - 2005 Na2S + 1/8S8 - 2005 Na2S2...
Na2S + 1/8S8 -> Na2S2...Na2S7}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/f6c118f479c3462fc2909c9fc80e573ab10e604e" style="vertical-align: -1.338ex; width:50.208ex; height:3.676ex;"/>
Because alkali metal sulfides are essentially salts of a weak acid and a strong base, they form basic solutions.
HS^- + HO^-}}}" xmlns="http://www.w3.org/1998/Math/MathML">S2− − +H2OΔ Δ HS− − +MAN− − {displaystyle {ce {S^2- + H2O - 2005 HS^- + HO^-}}}
HS^- + HO^-}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/10ba3bbadef64c0b8ee93810ff7cb0d8ffb9893c" style="vertical-align: -1.005ex; width:28.616ex; height:3.343ex;"/> H2S + HO^-}}}" xmlns="http://www.w3.org/1998/Math/MathML">HS− − +H2OΔ Δ H2S+MAN− − {displaystyle {ce {HS^- + H2O - 2005 H2S + HO^-}}
H2S + HO^-}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/2ea8c7ada247dd467375addf5be5896ebd4f30c4" style="vertical-align: -1.005ex; width:29.08ex; height:3.176ex;"/>
Reaction with nitrogen
Lithium is the only metal that combines directly with nitrogen at room temperature.
Li3N}}}" xmlns="http://www.w3.org/1998/Math/MathML">3Li+13N2Δ Δ Li3N{displaystyle {ce {3Li + 1/3N2}}}}
Li3N}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/e70dafabe2fd1d1f8ab8b5554db904b0b0e0e39f" style="vertical-align: -1.338ex; width:20.938ex; height:3.676ex;"/>
Li3N can react with water to liberate ammonia.
3LiOH + NH3}}}" xmlns="http://www.w3.org/1998/Math/MathML">Li3N+3H2OΔ Δ 3LiOH+NH3{displaystyle {ce {Li3N + 3H2O - 2005 3LiOH + NH3}}}}}}
3LiOH + NH3}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/5f5646ca6d5ba3bb5140cfe05640da9d7cb3a7ca" style="vertical-align: -1.005ex; width:33.57ex; height:2.843ex;"/>
Reaction with hydrogen
With hydrogen, the alkali metals form salt hydrides that hydrolyze in water.
NaH}}}" xmlns="http://www.w3.org/1998/Math/MathML">Na+H2Δ Δ NaH{displaystyle {ce {Na + H2 - 2005 Nah!
NaH}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/52e962c152975af97b8c7aa49d9e3309fa33820f" style="vertical-align: -1.005ex; width:18.288ex; height:2.843ex;"/> (high temperatures) NaOH + H2}}}" xmlns="http://www.w3.org/1998/Math/MathML">NaH+H2OΔ Δ NaOH+H2{displaystyle {ce {NaH + H2O-negative NaOH + H2}}}}
NaOH + H2}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/3e2b3d8fa85cf333877a84b8a48982961ad41872" style="vertical-align: -1.005ex; width:29.285ex; height:2.843ex;"/>
Reaction with carbon
Lithium is the only metal that reacts directly with carbon to give dilithium acetylide. Na and K can react with acetylene to give acetylides.
Li2C2}}}" xmlns="http://www.w3.org/1998/Math/MathML">2Li+2CΔ Δ Li2C2{displaystyle {ce {2Li + 2C - 2005 Li2C2}}}}
Li2C2}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/6192f816ae9119da54f8d801e93e3fdaef2a001a" style="vertical-align: -1.005ex; width:20.699ex; height:2.843ex;"/> NaC2H + 1/2H2}}}" xmlns="http://www.w3.org/1998/Math/MathML">Na+C2H2Δ Δ NaC2H+12H2{displaystyle {ce {Na + C2H2}{ NaC2H + 1/2H2}}
NaC2H + 1/2H2}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/25db42aaf404c571e1d2582cdff84d89a1796aba" style="vertical-align: -1.171ex; width:31.048ex; height:3.509ex;"/>
Reaction with water
By reacting with water, they generate hydroxide ions and hydrogen gas. This reaction is vigorous and highly exothermic and the resulting hydrogen can ignite in air or even explode in the case of Rb and Cs.
NaOH + 1/2H2}}}" xmlns="http://www.w3.org/1998/Math/MathML">Na+H2OΔ Δ NaOH+12H2{displaystyle {ce {Na + H2O) NaOH + 1/2H2}}
NaOH + 1/2H2}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/6cb7b2f2a4e575fe29996bf0c84cbf047b0520e5" style="vertical-align: -1.171ex; width:29.2ex; height:3.509ex;"/>
Reaction with other salts
Alkali metals are very good reducing agents. They can reduce metal cations that are less electropositive. Titanium is produced industrially by reducing titanium tetrachloride with Na at 400 °C (Van Arkel process).
4 NaCl + Ti}}}" xmlns="http://www.w3.org/1998/Math/MathML">TiCl4+4NaΔ Δ 4NaCl+Ti{displaystyle {ce {TiCl4 + 4 Na-give 4 NaCl + Ti}}}
4 NaCl + Ti}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/672da1c3feb50325296f0e767743041188de2101" style="vertical-align: -1.005ex; width:30.041ex; height:2.843ex;"/>
Reaction with organohalide compounds
Alkali metals react with halogen derivatives to generate hydrocarbons through the Wurtz reaction.
H3C - CH3 + 2 NaCl}}}" xmlns="http://www.w3.org/1998/Math/MathML">2CH3− − Cl+2NaΔ Δ H3C− − CH3+2NaCl{displaystyle {ce {2 CH3} - Cl + 2 Na - parent H3C - CH3 + 2 NaCl}}}}
H3C - CH3 + 2 NaCl}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/f9a61d917173e063c662f4de5fe0aeb2ca16ad83" style="vertical-align: -1.005ex; width:42.929ex; height:2.843ex;"/>
Alkali metals in liquid ammonia
Alkali metals dissolve in liquid ammonia or other donor solvents such as aliphatic amines or hexamethylphosphoramide to give blue solutions. These solutions are believed to contain free electrons.
Na+ + e(NH3)x-}}}" xmlns="http://www.w3.org/1998/Math/MathML">Na+xNH3Δ Δ Na++e(NH3)x− − {displaystyle {ce {Na + xNH3}{ Na+ + e(NH3)x}}}}}
Na+ + e(NH3)x-}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/65193f02f3adedde0944199baf25fb0305d2ad6e" style="vertical-align: -1.005ex; width:33.987ex; height:3.176ex;"/>
Due to the presence of solvated electrons, these solutions are very powerful reducing agents used in organic synthesis. Other reductions that can be achieved by these solutions are:
S8^2-}}}" xmlns="http://www.w3.org/1998/Math/MathML">S8+2e− − Δ Δ S82− − {displaystyle {ce {S8 + 2e-g) S8^2-}
S8^2-}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/eeb82e9a83125984504f143565ae154bf24db8bd" style="vertical-align: -1.005ex; width:18.001ex; height:3.343ex;"/>
Fe(CO)4^2- + CO}}}" xmlns="http://www.w3.org/1998/Math/MathML">Fe(CO)5+2e− − Δ Δ Fe(CO)42− − +CO{displaystyle {ce {Fe(CO)5 + 2e- - voluntary Fe(CO)4^2- + CO}}}
Fe(CO)4^2- + CO}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/f4b311253d80d1dfbd9f5867748f14cc113dc1db" style="vertical-align: -1.005ex; width:37.434ex; height:3.509ex;"/>
Extensions
Although francium is the heaviest alkali metal yet discovered, some theoretical work has been done that predicts the physical and chemical characteristics of hypothetical heavier alkali metals. Being the first element of period 8, the undiscovered element ununenium (element 119) is predicted to be the next alkali metal after francium and to behave much like its lighter congeners; however, it is also predicted to differ from the lighter alkali metals in some properties. Its chemistry is predicted to be closer to that of potassium or rubidium rather than cesium or francium. This is unusual since periodic trends ignoring relativistic effects would predict ununenium to be even more reactive than cesium and francium. This decreased reactivity is due to the relativistic stabilization of the valence electron of the ununennium, increasing the first ionization energy of the ununennium and decreasing the metallic and ionic radii; This effect is already seen for francium. This assumes that ununenium will behave chemically as an alkali metal, which, although likely, may not be true due to relativistic effects. The relativistic stabilization of the 8 orbital also increases the electron affinity of a unennium well beyond that of cesium and francium; in fact, ununenium is expected to have a higher electron affinity than all alkali metals lighter than it. Relativistic effects also cause a very large drop in the ununenium polarization. On the other hand, ununenium is predicted to continue the trend of lower melting points going down the group, and is expected to have a melting point between 0 °C and 30 °C.
The stabilization of the valence electron of ununennium and thus the contraction of the 8s orbital cause its atomic radius to be reduced to 240 pm, very close to rubidium (247 pm), so that the chemistry of ununennium in the +1 oxidation state is more similar to the chemistry of rubidium than to that of francium. On the other hand, the ionic radius of the Uue+ ion is predicted to be larger than that of Rb+, because the 7p orbitals are destabilized and therefore more larger than the p orbitals in the lower shells. Ununenium can also display the +3 oxidation state, which is not seen in any other alkali metal, in addition to the +1 oxidation state that is characteristic of the other alkali metals and is also the main oxidation state of all known alkali metals: This is due to destabilization and expansion of the 7p3/2 spinor, causing its outermost electrons to have a lower ionization energy than would otherwise be expected. In fact, many ununenium compounds are expected to be highly covalent in character, due to the involvement of the 7p3/2 electrons in the bonding.
Not as much work has been done to predict the properties of alkali metals beyond the ununenium. Although a simple extrapolation from the periodic table (according to the Aufbau Principle) would put element 169, a hexennium, under a unennium, Dirac-Fock's calculations predict that the next element after a unennium with alkali-like properties may be element 165, unhexpentium, which is predicted to have the electron configuration [Og] 5g18 6f14 7d10 8s2 8p 1/22 9s1. This element would have properties intermediate between an alkali metal and a group 11 element, and although its physical and atomic properties would be closer to the former, its chemistry might be closer to that of the latter. Other calculations show that unhexpentium would follow the trend of increasing ionization energy beyond cesium, which has comparable ionization energy to sodium, and that it should also continue the trend of decreasing atomic radii beyond cesium, with an atomic radius comparable to that of potassium. However, the 7d electrons of unhexpentium can also participate in chemical reactions along with the 9s electron, possibly allowing oxidation states beyond +1, hence the likely transition metal behavior of unhexpentium.
Because the alkali and alkaline earth metals are s-block elements, these predictions for the trends and properties of ununennium and unhexpentium also hold quite similarly for the corresponding alkaline earth metals unbinilium (Ubn) and unhexhexium (Uhh). Unsepttrium, element 173, may be an even heavier homologue of a unennium; with a predicted electron configuration of [Usb] 6g1, it returns to the alkali metal-like situation of having an electron that is easily removed well above an energy-closed p shell, and is expected which is even more reactive than cesium.
The probable properties of other alkali metals beyond unsepttrium have not yet been explored as of 2019, and may or may not exist. In periods 8 and above of the periodic table, relativistic and shell-structure effects become so strong that extrapolations from lighter congeners become completely inaccurate. Furthermore, the relativistic and shell structure effects (which stabilize the s orbitals and destabilize and expand the upper shell d, f, and g orbitals) have opposite effects, causing an even larger difference between relativistic and nonrelativistic. calculations of the properties of elements with such high atomic numbers. Interest in the chemical properties of ununenium, unhexpentium, and unsepttrium stems from the fact that they lie near the expected locations of the islands of stability, centered on elements 122 (306Ubb) and 164. (482Uhq).
Pseudo-alkali metals
Many other substances are similar to the alkali metals in their tendency to form monopositive cations. Analogously to the pseudohalogens, they have sometimes been called the 'pseudo-alkali metals'. These substances include some elements and many more polyatomic ions; Polyatomic ions are especially similar to alkali metals in their large size and weak polarizing power.
Hydrogen
The element hydrogen, with one electron per neutral atom, is usually placed at the top of Group 1 of the periodic table for convenience, but hydrogen is not normally considered an alkali metal; when it is considered to be an alkali metal, it is due to its atomic properties and not its chemical properties. Under typical conditions, pure hydrogen exists as a diatomic gas consisting of two atoms per molecule (H2); however, the alkali metals only form diatomic molecules (as dilithium, Li2) at high temperatures, when they are in a gaseous state.
Hydrogen, like the alkali metals, has one valence electron and reacts readily with halogens but the similarities end there due to the small size of a bare H+ proton compared to the halogens. alkali metal cations. Its placement above lithium is mainly due to its electronic configuration. It is sometimes placed above carbon due to their similar electronegativities or fluorine due to their similar chemical properties.
The first ionization energy of hydrogen (1312.0 kJ/mol) is much higher than that of the alkali metals. As only one additional electron is required to fill the outermost shell of the hydrogen atom, hydrogen often behaves like a halogen, forming the negative hydride ion, and is very occasionally considered a halogen on that basis. (Alkali metals can also form negative ions, known as alkalis, but these are little more than laboratory curiosities, being unstable.) An argument against this placement is that hydride formation from hydrogen is endothermic, as opposed to the exothermic formation of halides from halogens. The radius of the H- anion also does not fit the tendency of increasing the size of lowering the halogens: indeed, H- is very diffuse, since its single proton does not you can easily control both electrons. Liquid hydrogen had been expected for some time to show metallic properties; While this has been shown not to be the case, under extremely high pressures, such as those found in the cores of Jupiter and Saturn, hydrogen becomes metallic and behaves like an alkali metal; In this phase, it is known as metallic hydrogen. The electrical resistivity of liquid metallic hydrogen at 3000 K is approximately equal to that of liquid rubidium and cesium at 2000 K at the respective pressures when undergoing a nonmetal-to-metal transition.
The electron configuration of hydrogen 1s1, although superficially similar to that of the alkali metals (ns1), is unique in that there is no 1p subshell. Therefore, it can either lose an electron to form the hydron H+, or gain one to form the hydride ion H-. In the first case it superficially resembles the alkali metals; in the latter case, the halogens, but the differences due to the lack of a 1p subshell are large enough that neither group is a good fit for the properties of hydrogen. Group 14 also fits well in terms of thermodynamic properties, such as ionization energy and electron affinity, but does not make chemical sense because hydrogen cannot be tetravalent. Therefore, none of the three locations is completely satisfactory, although group 1 is the most common location (if one is chosen) because the hydron is by far the most important of all monatomic hydrogen species, being the basis of acid-base chemistry. As an example of the unorthodox properties of hydrogen stemming from its unusual electronic configuration and small size, the hydrogen ion is very small (radius around 150 fm compared to the 50-220 pm size of most other e atoms). ions) and therefore does not exist in condensed systems that are not associated with other atoms or molecules. In fact, the transfer of protons between chemicals is the basis of acid-base chemistry. Also unique is hydrogen's ability to form hydrogen bonds, which are an effect of contributing charge transfer, electrostatics, and electron correlative phenomena. While analogous lithium bonds are also known, they are mostly electrostatic. However, hydrogen can assume the same structural role as the alkali metals in some molecular crystals, and is closely related to the lighter alkali metals (especially lithium).
Ammonium and derivatives
The ammonium ion (NH+
4) has properties very similar to the heavier alkali metals, acting as an alkali metal intermediate between potassium and rubidium, and is often considered a close relative. For example, most alkali metal salts are soluble in water, a property shared by ammonium salts. Ammonium is expected to behave stably as a metal (NH+
4 ions in a delocalized electron sea) at very high pressures (although less than the typical pressure where transitions from insulating to metallic behavior occur, 100 GPa), and could possibly occur within the ice giants Uranus and Neptune, which may have an impact significant in their inner magnetic fields. It has been estimated that the transition from a mixture of ammonia and dihydrogen molecules to metallic ammonium can occur at pressures just below 25 GPa. Under standard conditions, ammonium can form a metallic amalgam with mercury.
Other "pseudo-alkali metals" they include alkylammonium cations, in which some of the hydrogen atoms in the ammonium cation are replaced by alkyl or aryl groups. In particular, quaternary ammonium cations (NR+
4) are very useful as they are permanently loaded, and are often used as an alternative to expensive Cs+ to stabilize very large and easily polarizable anions such as HI−
2 Tetraalkylammonium hydroxides, like the alkali metal hydroxides, are very strong bases that react with atmospheric carbon dioxide to form carbonates. Additionally, the nitrogen atom can be replaced with an atom of phosphorous, arsenic, or antimony (the heaviest non-metallic pycnogens), creating a phosphonium ( PH +
4) or arsonio (AsH+
4), a cation that can be substituted in a similar way.
Cobaltocene and derivatives
Cobaltocene, Co (C5H5)2, is a metallocene, the cobalt analogue of ferrocene. It is a dark purple solid. Cobaltocene has 19 valence electrons, one more than is usually found in organotransition metal complexes, like its very stable relative, ferrocene, according to the 18-electron rule. This extra electron occupies a bonding orbital with respect to Co-C bonds. Consequently, many chemical reactions of Co(C5H5)2 are characterized by their tendency to lose this "extra& electron #34;, producing a very stable 18-electron cation known as a cobaltocen. Many cobaltocenium salts coprecipitate with cesium salts, and cobaltocenium hydroxide is a strong base that absorbs atmospheric carbon dioxide to form cobaltocenium carbonate. Like the alkali metals, cobaltocene is a strong reducing agent, and decamethylcobaltocene is even stronger due to the combined inductive effect of the ten methyl groups. Cobalt can be substituted for its heavier congener rhodium to give rhodocene, an even stronger reducing agent. Iridocene (implying iridium) would presumably be even more potent, but it is not very well studied due to its instability.
Thallium
Thallium is the heaviest stable element in group 13 of the periodic table. At the bottom of the periodic table, the inert pair effect is quite strong, due to the relativistic stabilization of the 6s orbital and the decreasing binding energy as the atoms increase in size, so that the amount of energy released in the formation of two more bonds is not worth the high ionization energies of the 6s electrons. It shows the +1 oxidation state that all known alkali metals do, and thallium compounds with thallium in its +1 oxidation state closely resemble the corresponding potassium or silver compounds stoichiometrically due to the similar ionic radii. Tl+ (164 pm), K+ (152 pm) and Ag+ (129 pm) ions. It was sometimes considered an alkali metal in continental Europe (but not in England) in the years immediately following its discovery, and was placed just after cesium as the sixth alkali metal in Dmitri Mendeleev's 1869 periodic table and Julius, in Lothar Meyer's 1868 periodic table. (Mendeleev's 1871 periodic table and Meyer's 1870 periodic table put thallium in its present position in the boron group and left the space below cesium blank.) However, thallium also displays the +3 oxidation state, which no known alkali metal displays (although ununenium, the seventh undiscovered alkali metal, is predicted to possibly display the +3 oxidation state). The sixth alkali metal is now considered francium. While Tl+ is stabilized by the inert pair effect, this inert pair of 6s electrons can still participate chemically, such that these electrons are stereochemically active in aqueous solution. Furthermore, thallium halides (except TlF) are quite insoluble in water, and TlI has an unusual structure due to the presence of the stereochemically active inert pair on thallium.
Copper, silver and gold
The group 11 metals (or coinage metals), copper, silver, and gold, are generally classified as transition metals since they can form ions with incomplete D shells. Physically, they have the relatively low melting points and high electronegativity values associated with post-transition metals. Chemically, the group 11 metals behave like the main group metals in their +1 valence states, and are therefore somewhat related to the alkali metals: this is one reason they were previously labeled &# 34;group IB", in parallel with the alkali metals "group IA". They are sometimes classified as post-transition metals. Their spectra are analogous to those of the alkali metals. Its monopositive ions are paramagnetic and do not impart color to its salts, like those of the alkali metals.
In Mendeleev's 1871 periodic table, copper, silver, and gold are listed twice, once in group VIII (with the iron triad and platinum group metals), and once in group VIII. IB group. However, group IB is parenthesized to note that it was tentative. Mendeleev's main criterion for group assignment was the maximum oxidation state of an element: on that basis, group 11 elements could not be classified in group IB, due to the existence of copper(II) and gold compounds. (III) known at that time. hour. However, removing group IB would make group I the only main group (group VIII was labeled a transition group) that lacks an A–B bifurcation. Soon after, most chemists chose to classify these elements into group IB and remove them from group VIII for the resulting symmetry: this was the predominant classification until the rise of the modern 18-column, medium, and long periodic table, which separated the alkali metals and group 11 metals.
Coin metals were traditionally considered a subdivision of the alkali metal group, because they shared the s1 electronic configuration characteristic of the alkali metals (group 1: p6 s1; group 11: d10s1). However, the similarities are largely limited to the stoichiometries of the +1 compounds from both groups, and not to their chemical properties. This stems from the filled d subshell providing a much weaker shielding effect on the outermost s electron than the filled p subshell, so that minting metals have much higher first ionization energies and smaller ionic radii. than the corresponding alkali metals. In addition, they have higher melting points, hardnesses, and densities, and lower reactivities and solubilities in liquid ammonia, as well as having a more covalent character in their compounds. Finally, the alkali metals are at the top of the electrochemical series, while the coinage metals are almost at the bottom. The d-shell filled with coinage metals is much more easily altered than the p-subshell filled with alkali metals, so the second and third ionization energies are lower, allowing oxidation states higher than +1 and a richer coordination chemistry, giving group 11 Light Metals transition metal character. Particularly notable is gold that forms ionic compounds with rubidium and cesium, in which it forms the auride ion (Au-) which also occurs in a solvated form in liquid ammonia solution; here gold behaves like a pseudohalogen because its 5d10 6s1 configuration has one fewer electron than the quasi-closed 5d10 6s2 of mercury.
Production and isolation
The production of pure alkali metals is somewhat complicated due to their extreme reactivity with commonly used substances, such as water. From its silicate minerals, all stable alkali metals can be obtained in the same way: sulfuric acid is first used to dissolve the alkali metal ion and the desired aluminum(III) ions from the mineral (leaching)., whereby the basic precipitation removes the aluminum ions from the mixture, precipitating it as the hydroxide. The remaining insoluble alkali metal carbonate is then selectively precipitated; the salt dissolves in hydrochloric acid to produce the chloride. The result is allowed to evaporate and the alkali metal can be isolated. Lithium and sodium are usually isolated by electrolysis from their liquid chlorides, and calcium chloride is added to lower the melting point of the mixture. The heavier alkali metals, however, are more typically isolated in a different way, where a reducing agent (typically sodium for potassium and magnesium or calcium for the heavier alkali metals) is used to reduce the alkali metal chloride. The liquid or gaseous product (the alkali metal) is then subjected to fractional distillation for purification. Most routes to the pure alkali metals require the use of electrolysis due to their high reactivity; one of the few that is not is the pyrolysis of the corresponding alkali metal azide, which produces the metal for sodium, potassium, rubidium, and cesium and the nitride for lithium.
Lithium salts have to be extracted from water from mineral sources, salt flats, and brine deposits. The metal is produced electrolytically from a mixture of molten lithium chloride and potassium chloride.
Sodium occurs mainly in seawater and on dry seabeds, but is now produced through the electrolysis of sodium chloride by lowering the melting point of the substance to less than 700 °C through the use of a Downs cell. Extremely pure sodium can be produced through the thermal decomposition of sodium azide. Potassium is found in many minerals, such as sylvite (potassium chloride). Previously, potassium was usually made from the electrolysis of potassium chloride or potassium hydroxide, found widely in places like Canada, Russia, Belarus, Germany, Israel, the United States, and Jordan, in a method similar to how sodium was produced. in the late 1800s and early 1900s. It can also be produced from seawater. However, these methods are problematic because potassium metal tends to dissolve in its molten chloride and vaporizes significantly at operating temperatures, potentially forming the explosive superoxide. As a result, pure potassium metal is now produced by reducing molten potassium chloride with sodium metal at 850°C.
<math alttext="{displaystyle {ce {Na (g) + KCl(I) NaCl(I) + K(g)}}}" xmlns="http://www.w3.org/1998/Math/MathML">Na(g)+KCl(I) − − − − NaCl(I)+K(g){displaystyle {ce {Na (g) + KCl(I)} ≤ purported NaCl(I) + K(g)}}}}<img alt="{displaystyle {ce {Na (g) + KCl(I) NaCl(I) + K(g)}}}" aria-hidden="true" class="mwe-math-fallback-image-inline" src="https://wikimedia.org/api/rest_v1/media/math/render/svg/3c11402da94c88461e6e6d894fed34cbecfee85a" style="vertical-align: -0.838ex; width:35.647ex; height:3.176ex;"/>
Although sodium is less reactive than potassium, this process works because at such high temperatures potassium is more volatile than sodium and can easily be distilled off, so the equilibrium shifts to the right to produce more of the gas. potassium and almost complete.
For several years in the 1950s and 1960s, a byproduct of potassium production called Alkarb was a primary source of rubidium. The Alkarb contained 21% rubidium, while the rest was potassium and a small fraction of cesium. Today, the largest producers of cesium, for example the Tanco mine in Manitoba, Canada, produce rubidium as a byproduct of pollucite. Today, a common method for separating rubidium from potassium and cesium is the fractional crystallization of a rubidium-cesium alum (Cs, Rb) Al(SO4)2 12 H2O, yielding alum of pure rubidium after approximately 30 recrystallizations. Limited applications and the lack of a rubidium-rich ore limit the production of rubidium compounds to 2–4 tons per year. Cesium, however, is not produced from the above reaction. Instead, mining pollucite ore is the main method of obtaining pure cesium, extracted from ore mainly by three methods: acid digestion, alkaline decomposition, and direct reduction. Both metals are produced as by-products of lithium production: after 1958, when interest in lithium's thermonuclear properties increased sharply, rubidium and cesium production also increased correspondingly. The pure rubidium and cesium metals are produced by reducing their chlorides with metallic calcium at 750 °C and low pressure.
As a result of its extreme rarity in nature, most of the francium is synthesized in the nuclear reaction 197Au+18O → 210 Fr + 5n, producing francium-209, francium-210, and francium-211. The largest amount of francium ever assembled to date is approximately 300,000 neutral atoms, which were synthesized using the nuclear reaction given above. When the only natural isotope of francium-223 is specifically required, it is produced as the alpha daughter of actinium-227, itself produced synthetically from neutron irradiation of natural radium-226, one of the daughters of uranium- 238 natural.
Applications
Lithium, sodium, and potassium have many applications, while rubidium and cesium are very useful in academic contexts, but don't have many applications yet. Lithium is often used in lithium-ion batteries, and lithium oxide can help process silica. Lithium stearate is a thickener and can be used to make lubricating greases; It is produced from lithium hydroxide, which is also used to absorb carbon dioxide in space capsules and submarines. Lithium chloride is used as a brazing alloy for aluminum parts. Metallic lithium is used in alloys with magnesium and aluminum to give very strong and lightweight alloys.
Sodium compounds have many applications, the best known being sodium chloride as table salt. The sodium salts of fatty acids are used as soap. Pure sodium metal also has many applications, including use in sodium vapor lamps, which produce very efficient light compared to other types of lighting, and can help smooth the surface of other metals. Being a strong reducing agent, it is often used to reduce many other metals, such as titanium and zirconium, from their chlorides. Furthermore, it is very useful as a heat exchange fluid in fast breeder nuclear reactors due to its low melting point, viscosity, and cross section toward neutron absorption.
Potassium compounds are often used as fertilizers since potassium is an important element for plant nutrition. Potassium hydroxide is a very strong base and is used to control the pH of various substances. Potassium nitrate and potassium permanganate are often used as powerful oxidizing agents. Potassium superoxide is used in breathing masks as it reacts with carbon dioxide to give potassium carbonate and oxygen gas. The pure metal potassium is not often used, but its alloys with sodium can be substituted for pure sodium in fast breeder nuclear reactors.
Rubidium and cesium are often used in atomic clocks. Cesium atomic clocks are extraordinarily accurate; if a clock had been made at the time of the dinosaurs, it would be off by less than four seconds (after 80 million years). For that reason, cesium atoms are used as the definition of the second. Rubidium ions are often used in purple fireworks, and cesium is often used in drilling fluids in the petroleum industry.
Francium has no commercial applications, but due to francium's relatively simple atomic structure, among other things, it has been used in spectroscopy experiments, leading to more information about energy levels and energy constants. coupling between subatomic particles. Studies of the light emitted by laser-trapped francium-210 ions have provided precise data on transitions between atomic energy levels, similar to those predicted by quantum theory.
Biological role and precautions
Metals
Pure alkali metals are dangerously reactive with air and water and should be kept away from heat, fire, oxidizing agents, acids, most organic compounds, halocarbons, plastics, and moisture. They also react with carbon dioxide and carbon tetrachloride, so normal fire extinguishers are counterproductive when used on alkali metal fires. Some Class D dry powder extinguishers designed for metal fires are effective, starving the fire of oxygen and cooling the alkaline metal.
Experiments are usually performed using only small amounts of a few grams in a fume hood. Small amounts of lithium can be removed by reaction with cold water, but the heavier alkali metals must be dissolved in the less reactive isopropanol. Alkali metals should be stored in mineral oil or in an inert atmosphere. The inert atmosphere used can be argon or nitrogen gas, with the exception of lithium, which reacts with nitrogen. Rubidium and cesium must be kept out of air, even under oil, because even a small amount of air diffused into the oil can trigger the formation of the dangerously explosive peroxide; For the same reason, potassium should not be stored under oil in an oxygen-containing atmosphere for more than 6 months.
Ions
The bioinorganic chemistry of alkali metal ions has been extensively reviewed. Solid-state crystal structures have been determined for many alkali metal ion complexes in small peptides, nucleic acid constituents, carbohydrates, and ionophore complexes.
Lithium naturally occurs only in trace amounts in biological systems and has no known biological role, but does have effects in the body when ingested. Lithium carbonate is used as a mood stabilizer in psychiatry to treat bipolar disorder (manic depression) in daily doses of approximately 0.5 to 2 grams, although there are side effects. Excessive ingestion of lithium causes drowsiness, slurred speech, and vomiting, among other symptoms, and poisons the central nervous system, which is dangerous as the required dose of lithium to treat bipolar disorder is only slightly lower than the toxic dose. Its biochemistry, the way it is handled by the human body, and studies in rats and goats suggest that it is an essential trace mineral, although lithium's natural biological function in humans has yet to be identified.
Sodium and potassium occur in all known biological systems, generally functioning as electrolytes inside and outside cells. Sodium is an essential nutrient that regulates blood volume, blood pressure, osmotic balance, and pH; The minimum physiological requirement for sodium is 500 milligrams per day. Sodium chloride (also known as common salt) is the main source of sodium in the diet, and is used as a seasoning and preservative, such as for pickles and jerky; Most of it comes from processed foods. The dietary reference intake for sodium is 1.5 grams per day, but most people in the United States consume more than 2.3 grams per day, the minimum amount that promotes hypertension; This in turn causes 7.6 million premature deaths worldwide.
Potassium is the main cation (positive ion) inside animal cells, while sodium is the main cation outside animal cells. Differences in the concentration of these charged particles cause a difference in electrical potential between the inside and outside of cells, known as the membrane potential. The balance between potassium and sodium is maintained by ion transport proteins in the cell membrane. The cell membrane potential created by potassium and sodium ions allows the cell to generate an action potential, a "spike" of electric shock. The ability of cells to produce electrical discharges is critical to bodily functions such as neurotransmission, muscle contraction, and heart function. Disruption of this balance can be fatal: for example, ingestion of large amounts of potassium compounds can lead to hyperkalemia that strongly influences the cardiovascular system. Potassium chloride is used in the United States for lethal injection executions.
Because of their similar atomic radii, rubidium and cesium mimic potassium in the body and are absorbed in a similar way. Rubidium has no known biological role, but it can help stimulate metabolism, and, similar to cesium, it replaces potassium in the body causing potassium deficiency. Partial replacement is quite possible and quite non-toxic: a 70 kg person contains on average 0.36 g. of rubidium, and an increase in this value of 50 to 100 times did not show negative effects in the test persons. Rats can survive up to 50% substitution of potassium for rubidium. Rubidium (and to a much lesser extent cesium) may work as a temporary cure for hypokalemia; While rubidium can adequately replace potassium physiologically in some systems, cesium never can. There is only very limited evidence in the form of deficiency symptoms that rubidium is possibly essential in goats; even if this is true, the trace amounts generally present in food are more than enough.

Most people rarely encounter cesium compounds, but most cesium compounds are mildly toxic. Like rubidium, cesium tends to substitute for potassium in the body, but it is significantly larger and therefore a poorer substitute. Excess cesium can lead to hypokalemia, arrhythmia, and acute cardiac arrest, but such amounts would not normally be found in natural sources. As such, cesium is not a significant environmental chemical pollutant. The median lethal dose (LD50) value for cesium chloride in mice is 2.3 grams per kilogram, which is comparable to LD50 values for cesium chloride. potassium and sodium chloride. Cesium chloride has been promoted as an alternative cancer therapy, but has been linked to the deaths of more than 50 patients, in whom it was used as part of a scientifically unvalidated cancer treatment.
Radioisotopes of cesium require special precautions; Improper handling of cesium-137 gamma ray sources can lead to the release of this radioisotope and radiation injury. Perhaps the best-known case is the 1987 Goiânia accident, in which a poorly disposed radiotherapy system from an abandoned clinic in the city of Goiânia, Brazil, was removed from a junkyard, and the glowing cesium salt was sold. to curious, uneducated buyers. This led to four deaths and serious injuries from radiation exposure. Along with cesium 134, iodine 131, and strontium 90, cesium 137 was among the isotopes released by the Chernobyl disaster that pose the greatest health risk. Presumably, francium radioisotopes would also be dangerous due to their high decay energy and short half-life, but none have been produced in quantities large enough to pose a serious risk.
Contenido relacionado
Sea salt
Coordination link
Potassium nitrate