Aldehyde
The aldehydes are organic compounds characterized by having the functional group -CHO (carbonyl). A carbonyl group is obtained by separating a hydrogen atom from formaldehyde. As such it does not have free existence, although all aldehydes can be considered to possess a terminal carbonyl group.
Aldehydes are named after the corresponding alcohols, changing the ending -ol to -al. Etymologically, the word aldehyde comes from the scientific Latin alcohol dehydrogarium (dehydrogenated alcohol).
Properties
Physical Properties
- The double union of the carbonyl group since the carbonyl group is polarized due to the resonance phenomenon.
- Hydrogen aldehydes on a sp3 carbon in alpha position to the carbonyl group present tautomeric isomery. The aldehydes are obtained from the dehydration of a primary alcohol with potassium permanganate, the reaction has to be weak, the ketones are also obtained from the dehydration of an alcohol, but these are obtained from a secondary alcohol and are equally dehydrated with potassium permanganate and obtained with a weak reaction, if the alcohol reaction is strong, the result will be a strong acid.
Chemical Properties
- They behave as a reducer, by oxidation the aldehyde gives acids with equal number of carbon atoms.
- The typical reaction of aldehydes and ketones is the nucleophilic addition.
Nomenclature
They are named by replacing the ending -ol of the name of the alcohol with -al. The simpler aldehydes (methanal and ethanal) have other names that do not follow the International Union of Pure and Applied Chemistry (IUPAC) standard but formaldehyde and acetaldehyde are more commonly used, respectively, the latter two being trivial names accepted by IUPAC. The homologous series for the following aldehydes is: H-(CH
2)
n-CHO (n = 0, 1, 2, 3, 4, …)
| Number of carbons | Nomenclature IUPAC | Trivial nomenclature | Formula | P.E.°C |
|---|---|---|---|---|
| 1 | Target | Formaldehído | HCHO | -21 |
| 2 | Etanal | Acetaldehyde | CH 3CHO | 20.2 |
| 3 | Propanal | Propionaldehyde Propilaldehído | C 2H 5CHO | 48.8 |
| 4 | Butanal | n-Butiraldehído | C 3H 7CHO | 75.7 |
| 5 | Pentanal | n- Valeraldehydo. Amilaldehydo n-Pentaldehído | C 4H 9CHO | 103 |
| 6 | Hexanal | Capronaldehyde n-Hexaldehydo. | C 5H 11CHO | 100.2 |
| 7 | Heptanal | Enantaldehí Heptilaldehyde n-Heptaldehyde. | C 6H 13CHO | 48.3 |
| 8 | Eighth | Caprilaldehído n-Octilaldehído | C 7H 15CHO | Between 55,381 and 55,389 (approximately) |
| 9 | Nonanal | Pelargonaldehyde n-Nonilaldehído | C 8H 17CHO | 62.47 |
| 10 | Decanal | Caprinaldehyde n- Decilaldehydo. | C 9H 19CHO | 10.2 |
To name aldehydes as substituents
If it is a substituent of a substituent
Aldehydes are terminal functions, that is, they go to the end of chains Cycle nomenclature
| Logger | Main Carbon Chain | Carbaldehido | Example |
|---|---|---|---|
| 1(can be omitted) | Benceno | Carbaldehido | 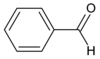 |
| 2.3 | Naphthalene | DiCarbaldehido |  |
If the cycle has other less important substituents, they are named first, like this:
For further details see aldehyde nomenclature
Reactions of aldehydes
Aromatic aldehydes such as benzaldehyde dismutate in the presence of a base giving the alcohol and the corresponding carboxylic acid:
2 C
6H
5CHO → C
6H
5CH
2OH + C
6H
5CO
2H
With primary amines they give the corresponding imines in an exothermic reaction that is often spontaneous:
R-CH=O + H
2N-R' → R-CH=N-R'
In the presence of reducing substances such as some hydrides or even other aldehydes they can be reduced to the corresponding alcohol while strong oxidants transform them into the corresponding carboxylic acid.
With ketones that carry a hydrogen on an sp³ carbon in the presence of acidic or basic catalysts, aldol-type condensations are produced.
With alcohols or thiols in the presence of hygroscopic substances, acetals can be obtained by condensation. As the reaction is reversible and the aldehydes are recovered in an acid medium and in the presence of water, this reaction is used to protect the functional group.
Summary
- By oxidation of primary alcohols
They can be obtained from the soft oxidation of primary alcohols. This can be done by heating the alcohol in an acid solution of potassium dichromate (there are also other methods that use Cr in the +6 oxidation state). Dichromate reduces to Cr3+
(green). Also by Swern oxidation, using dimethyl sulfoxide, (DMSO), oxalyl dichloride, (CO)
2Cl
2, and a base. Schematically the oxidation process is as follows:
- For charcoal.
- By alkylo halogenide oxidation (Oxidation of Kornblum)
- By reduction of carboxylic acids or their derivatives (esters, alkylo halogenides).
Synthesis of aromatic aldehydes
- Gattermann-Koch reaction
- Hoesch reaction
- Reimer-Tiemann Reaction
- Vilsmeier-Haack reaction
Uses
Aldehydes are used mainly for the manufacture of resins, plastics, solvents, paints, perfumes, essences.
Aldehydes are present in numerous natural products and great varieties of them are from our own daily life. Glucose, for example, exists in an open form that has an aldehyde group. Acetaldehyde formed as an intermediate in metabolism is believed to be largely responsible for hangover symptoms after ingesting alcoholic beverages.
Formaldehyde is a preservative found in some compositions of cosmetic products. However, this application must be viewed with caution since in animal experiments the compound has shown carcinogenic power. It is also used in the manufacture of numerous chemical compounds such as bakelite, melamine, etc.
Contenido relacionado
Radioisotope
Meteorology
Nucleoside


