Activated carbon

Activated carbon or active carbon is a generic term that describes a family of highly crystalline carbonaceous adsorbents with highly developed internal porosity.
There is a wide variety of activated carbon products that show different characteristics, depending on the starting material and the activation technique used in their production.
It is a material that is characterized by having a very large number of micropores (pores less than 2 nanometers in radius). Because of its high microporosity, carbon can have a surface area of 50 m²/g or more if it is active, reaching values of more than 2500 m²/g.
Activated carbon is used in the extraction of metals (e.g. gold), the purification of drinking water (both for public and domestic purification), in Veterinary Medicine and Human Medicine for cases of poisoning, in wastewater treatment, sugar syrup clarification, glycerin purification, in gas masks, in purification filters and in automobile emissions controllers, among many other uses.
Production
It is generally produced by two different methods:
- Chemical activation: a dehydrating substance, which can be an acid, is mixed with raw material and subjected to moderate temperature treatment. This technique can be problematic because, for example, by using zinc chloride dehydrating agent zinc residues can remain in the final product, even after washing.
- Physical or steam activation: the carbonized material is treated with a mixture of combustion gases and water vapor at a high temperature to be activated.
Various carbonaceous materials are used as starting materials, such as nutshells, wood, coconut.
Features

Activated carbon can have a surface area greater than 500 m²/g, with values of 1000 m²/g being easily achievable. Some activated carbons can reach values greater than 2500 m²/g. For comparison, a tennis court is about 260 m².
Under an electron microscope, the structure of activated carbon shows up with a large number of nooks and cracks. At lower levels there are areas where there are small flat graphite-like surfaces, separated only by a few nanometers, forming micropores. These micropores provide the conditions for the adsorption process to take place. The evaluation of adsorption is generally done using nitrogen gas at 77 K under high vacuum conditions.
Saturated activated carbon can be regenerated by applying heat. Carbon aerogels, which are more expensive, have very high effective surface areas and find similar use to activated carbon in special applications.
Its main characteristics are:
Surface area
It is the extension of the pore surface developed within the activated carbon matrix. It is measured using nitrogen (N2). It is the primary indicator of the activity level, assuming that the greater the surface area, the greater the number of adsorption points available.
Poral radios
Determining the distribution of pore sizes is an extremely useful way to know the behavior of the material. The IUPAC defines the distribution of pore rays as follows:
- Microporos r ≤2 nm
- Mesoporos r ≈ 2-50 nm
- Macroporos r 한 50 nm
The macropores are the entry route to the activated carbon, the mesopores carry out the transport, and the micropores carry out the adsorption.
Iodine number
It is a measurement of porosity by adsorption of iodine in solution.
Activity of carbon tetrachloride
It is a measure of porosity by absorption of saturated vapor of carbon tetrachloride.
Hardness
Hardness is an important factor in system design, filter life and handling. It presents great variations, depending on the original material and its level of activity.
Density
Bulk density should be carefully considered when fixed volumes must be filled; may have commercial implications.
The cleaned and dried density always shows a lower value, due to the water film between the activated carbon particles.
Particle size
The finer the particle size of a given activated carbon, the better the access to surface area and the faster the rate of absorption kinetics. In vapor phase systems, this must be considered along with pressure drop, which affects energy costs.
Careful choice of particle size can provide significant operational benefits.
Process of obtaining activated carbon
The activated carbon process is based on producing charcoal from materials such as: almond bark, coconut shell, peat, oil, tar and polymers, nuts, palm trees or other woods, and mineral charcoal.
This process can be divided into two types:
- Physical activation (thermal). It is carried out in two stages, the carbonization that eliminates elements such as hydrogen and oxygen to give rise to a rudimentary porous structure and the gasification stage of the carbonized that is exposed to an oxidizing atmosphere that eliminates volatile products and carbon atoms, increasing the volume of pores and the specific surface. This is done in different ovens at temperatures close to 1000 °C.
- Chemical activation. The material is impregnated with a chemical agent that can be phosphoric acid or potassium hydroxide and heated in an oven at 500-700 °C. Chemical agents reduce the formation of volatile material and alchetrans, increasing carbon performance. The resulting is washed for the elimination of acid.
The type of material from which activated carbon is produced affects the pore size and regeneration characteristics of the activated carbon. The two types of classification are: powder activated carbon, with a diameter less than or equal to 0.25mm, and granular carbon, with a diameter greater than 0.25mm. Its surface area and pore volume make it highly competitive in the search for green technologies.
For example, orange peel, being a lignocellulosic material, consists of a certain percentage of moisture, therefore, carbonization must adjust drying to a certain time to obtain this material. The methods for the production or obtaining of these natural materials usually occur by chemical or physical impregnation. The purpose of these materials is based on the adsorption of gases due to the pore size, its pore with a radius between 2-50 nm.
The sizes of the pores range from the smallest, called micropores (up to 2.0 nm), to mesopores (2.0 to 50 nm) and macropores (greater than 50 nm).
Thermal reactivation

Saturated activated carbon can be thermally reactivated through a high temperature process (up to 900 °C), for example in rotary kilns or multistage kilns. Thanks to modern and efficient gas treatments, activated carbon saturated, coming from different applications, can be recycled.
The entire reactivation process includes the following steps:
- Drying material up to ± 105 °C.
- Evaporation of the adsorbed volatile compounds up to ± 300 °C.
- Non-volatile compounds adsorbed within active coal are decomposed tomorphic carbon in the atmosphere of the oven through a pyrolysis process with temperatures up to ± 600 °C.
- Finally, the charcoal in amorphous form is gassed by the injection of steam at high pressure and temperature (over 800 °C).
During the reaction of amorphous carbon with high-temperature steam to carbon monoxide (CO) and, subsequently, carbon dioxide (CO2), micropores are formed that will give the active carbon a large specific surface area.
Through the rational use of natural resources, the reactivation of active carbon reduces CO2 emissions to the atmosphere by 80%.
Applications
Medical use
Activated charcoal is used as an adsorbent agent to treat poisonings and overdoses due to oral ingestion. Prevents the absorption of the toxic substance in the stomach. The typical dosage for an adult is 1 g/kg within the first hour of poisoning, with a maximum limit of 100 g total. Pediatric doses are 12-25 g (1 g/kg). For drugs with enterohepatic circulation such as carbamazepine, digoxin, morphine, among others, it is suggested to administer activated charcoal in multiple doses (0.5 g/kg every 4 hours for 24 to 48 hours).
If possible, combine with some juice or flavored liquid to avoid the bad taste of activated charcoal and so the patient does not vomit it. Incorrect use of this product can cause bronchoaspiration (entry into the lungs) and can lead to a fatal outcome if not controlled. For use outside the hospital, it comes in 1 g tablets, or in tubes or plastic bottles, commonly 12.5 or 25 g, premixed with water. It has trade names like InstaChar, SuperChar, Actidose, and Liqui-Socarra, but it is usually simply called activated charcoal.
During recent years, activated charcoal has become a "magical remedy" to lose weight; a supposedly detox food that has come to the news spotlight and has become a real fad with the consequent dangers that this implies. In fact, the adsorption capacity of activated charcoal is unable to distinguish between harmful and beneficial substances, so it is possible that it ends up blocking the absorption of certain vitamins and minerals that the body needs to function with frequent consumption. Excessive consumption can also cause constipation, intestinal blockages and other more serious problems. However, the European Community and the American FDA consider it as a food supplement.
Other of its most widespread uses is teeth whitening. However, there is no scientific evidence that it is beneficial for this purpose and, in fact, experts suggest that the whitening is due to the abrasion of the microparticles on the enamel. These - like the substances contained in other home remedies such as baking soda or lemon - weaken the structure of the tooth and can cause permanent damage to the dentin if used regularly.
Filters for air, compressed gas and purifying water
Filters with activated carbon are generally used in the purification of air, water and gases, to remove oil vapors, flavors, odors and other hydrocarbons from air and compressed gases.
The most common designs use single- or two-stage filters, where activated carbon is introduced as a filter medium. An example could be the filter that cigarettes have. It is also used for purifying rainwater in areas where it is used for domestic purposes.
For its application in water treatment, 1 to 3 cubic feet (approximately 30 to 90 cubic decimeters) of activated carbon are required to treat 1 million liters of water, as long as the free chlorine concentration is equal to or less than 1 ppm (part per million).
There are activated carbon filters to which silver is added so that bacteria do not develop in it, according to the antiviral and antibacterial properties of colloidal silver.[citation required ] Filters with smaller activated carbon particles generally have a better adsorption rate.
On the other hand, the acidity and temperature of the water to be filtered influence the behavior of the activated carbon filter. At higher acidity and lower water temperatures, the performance of activated carbon filters improves. Asbestos cannot be removed from water through an activated carbon filter.
An activated carbon filter must be replaced between every 2,800 and 3,750 liters of filtered water, which is only a reference since the filtration capacity and life of the filter will depend on the quality of the water that is filtered. The pore size of the activated carbon and the size of the particles to be filtered also influence the life and filtration capacity of the activated carbon filter.
So the only way to know if an activated carbon filter has stopped working is to do an analysis of the water resulting from the filter, since neither the taste nor smell can be an accurate reference. Once an activated carbon filter has been saturated, the water that passes through it will be more contaminated than if it were not filtered.
Activated carbon filters that are placed at the end of the faucet have inferior performance compared to those placed under the sink or sink due to the small volume of activated carbon they contain. It is also recommended to replace activated carbon filters at twice the rate recommended by manufacturers. The filters that "warn" The time to change the filter is inaccurate and saturation and consequent contamination of the water can occur long before warning.[citation needed]
Catalyst agent
Activated carbon is a very versatile adsorbent due to its distribution and pore size. Its main application is in the elimination of impurities from gases and liquids; It has been established that said elimination takes place through an adsorption process. The surface of the activated carbon can trap the molecules of the gas or liquid phase through predominantly physical forces (Van der Waals type), causing a high concentration on its surface.
A clear example of a reaction through the implementation of activated carbon is the hexaaminocobalt(III) complex ([Co(NH3)6]3+) which is thermodynamically unstable in acidic solutions and its reaction equilibrium constant borders the 1x1020. At equilibrium, the concentration of [Co(NH3)6]3+ is very low so the reaction can take days to complete, that is, it is an inert complex that undergoes very slow change reactions. Due to its characteristics, the complex has a very low concentration, so adding active carbon will favor the concentration of the complex to considerably reduce the reaction time, consequently accelerating the process without altering its chemical properties.
- Acid catalysis
They are excellent acid catalysts since they are easy to convert with a sustainable economic investment. It is oxidized in the presence of water, favoring the formation of oxygenated and hydrogenated groups (carboxyls). In this type of catalysis it is common for the specific surface area of the activated carbon to decrease without affecting the catalysis.
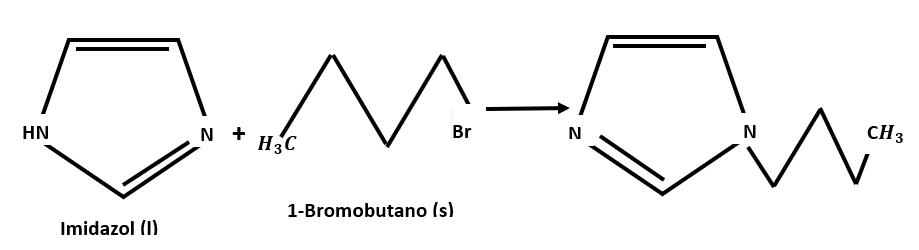
The point of zero charge (PCC) of these materials depends on the original activated carbon and the acid used, but not on the number of existing acidic functional groups, demonstrating that the type of functional group is more important than their number. One of these reactions has been the alkylation of nitrogenous heterocycles.

- Basic catalysis
Catalysis in this case usually has an alkaline action. Activated carbon is put into action with a solution of an alkali metal salt corresponding to the cations (Na, K and Cs), which causes the carbon to undergo little variation in its physical structure, but not in its chemical properties.
The heavier the alkaline element, the greater the basicity of the catalyst, which is directly related to its catalytic activity.
Environmental uses
The adsorption properties of activated carbon are very useful in removing contaminants from the air and from water flows involved in industrial processes:
- Cleaning of discharges
- Recovery of surface and groundwater
- Drinking water treatment
- Air purification
- Collection of volatile compounds from industrial processes such as painting, dry cleaning, fuel refueling...
- Water purification not intended for human consumption
- Purification of amino acids
- Creation of homemade organic fertilizer.
- Component for the creation of recycled paper.
- Purification of ingredients and food products
- Separation and purification of gases such as biogas, carbon dioxide, hydrogen, synthesis gas.
- Personal and collective protection (e.g. gas masks)
Contenido relacionado
Ammonium
Donald glaser
Endemism
