Actin
Actin is a family of proteins that make up microfilaments, one of the three fundamental components of the cytoskeleton of cells of eukaryotic organisms (also called eukaryotes). It can be found as a monomer in free form, called G actin, or as part of linear polymers called microfilaments or F actin, which are essential for functions cell functions as important as cell mobility and contraction during cell division.
The fundamental importance of actin is evidenced by the fact that it always represents a high percentage of the protein content of a cell and that its sequence is highly conserved, that is, it has changed very little throughout evolution. For both reasons, it can be said that its structure has been optimized. Two peculiar features can be highlighted about this: it is an enzyme that hydrolyzes ATP, the "universal currency of energy" of biological processes, doing it very slowly. But at the same time it needs that molecule to maintain its structural integrity. It acquires its effective form in an almost dedicated folding process. It is also the one that establishes more interactions with other proteins than are known, which allows it to perform the most varied functions that affect almost all aspects of cellular life. Myosin is an example of an actin-binding protein. Another example is villin, which can entwine actin into bundles or cleave actin filaments, depending on the concentration of calcium cation in its environment.
Forming microfilaments in a dynamic process provides a scaffolding that provides the cell with a shape with the ability to rapidly remodel itself in response to its environment or signals from the organism, for example, by increasing cell surface area for absorption or providing support for adhesion of cells to form tissues. Other enzymes, organelles such as the cilium, can be anchored on this scaffolding, directing the deformation of the outer cell membrane that allows cell ingestion or cytokinesis. It can also produce movement, either by itself or with the help of molecular motors. Thus, it contributes to processes such as intracellular transport of vesicles and organelles and muscle contraction, or cell migration, important in embryonic development, wound repair, or cancer invasiveness. The evolutionary origin of this protein can be traced to prokaryotic cells, where equivalents exist. Finally, it is important in the control of gene expression.
A good number of diseases are based on genetic alterations in alleles of the genes that govern the production of actin or its associated proteins, being also essential in the infection process of some pathogenic microorganisms. Mutations in the various actin genes present in humans cause myopathies, variations in cardiac size and function, and deafness. The components of the cytoskeleton are also related to the pathogenicity of intracellular bacteria and viruses, especially in processes related to the evasion of the immune system response.
History
Actin was first observed experimentally in 1887 by W.D. Halliburton, who extracted a muscle protein that coagulated myosin preparations, naming it "myosin ferment". However, Halliburton was unable to characterize his observations, and the discovery is therefore attributed to Brúnó F. Straub, then a young biochemist who worked in the laboratory of Albert Szent-Györgyi at the Institute of Medicinal Chemistry at the University of Szeged, in Hungary.
In 1942, Straub developed a new technique for muscle protein extraction that enabled him to isolate substantial amounts of relatively pure actin. This method is essentially the same as that used in laboratories today. Szent-Györgyi had previously described a more viscous form of myosin, produced by slow extractions in muscle, as "activated myosin," and since Straub's protein produced the activating effect, he named it actin. The viscosity decreased if ATP was added to the mixture of both proteins, known as actomyosin. Their work could not be published in Western countries due to the warlike environment of World War II, coming to light in 1945 when it was published as a supplement to Acta Physiologica Scandinavica. Straub continued to work on actin until 1950, publishing that it could bind to ATP and that, during protein polymerization to form microfilaments, it hydrolyzed to ADP + Pi, which remained attached to the microfilament. Straub suggested that this reaction played a role in muscle contraction, but this is only true for smooth muscle and was not verified experimentally until 2001.
The amino acid sequence was completed by Elzinga et al. in 1973, and the crystallographic structure of G-actin was determined in 1990 by Kabsch et al., although it was a cocrystal complexed with deoxyribonuclease I, a model being proposed the same year for F-actin by Holmes and his collaborators. This co-crystallization procedure with different proteins was used repeatedly during the following years, until in 2001 the isolated protein was crystallized together with ADP. It was possible thanks to the use of a rhodamine conjugate that prevented polymerization by blocking the cys-374 amino acid. ABP's. The crystals turned out to be too small for the technology of the time.
Although there is currently no high-resolution model of the filamentous form, Sawaya's team in 2008 made a more accurate approximation based on multiple actin dimer crystals contacting at different locations. This model was refined by Sawaya himself. author and by Lorenz. Other approaches, such as the use of cryoelectron microscopy or synchrotron radiation, have recently made it possible to increase the level of resolution and better understand the nature of the interactions and conformational changes involved in actin filament formation.
Structure
Actin is one of the most abundant proteins among eukaryotes and is present throughout the cytoplasm. In fact, in muscle fibers it represents 20% by weight of total cell protein and, in other animal cells, it ranges between 1 and 5%. However, there is not a single type of actin, but rather its coding genes are defined by a multigenic family (a family that, in plants, contains more than 60 elements, between genes and pseudogenes and, in humans, more than 30). This means that each individual's genetic information contains instructions for generating actin variants (called isoforms) that will have slightly different functions. In this way, eukaryotic organisms express different genes that give rise to: α-actin, which is found in contractile structures; β-actin, at the expanding edge of cells that use the projection of cell structures as a method of mobility; and γ-actin, in the filaments of stress fibers. In addition to the similarities between isoforms within an organism, there is also evolutionary conservation in structure and function between organisms from even non-eukaryotic domains: in bacteria, The MreB homologue is known, a protein that is capable of polymerizing in microfilaments; and in archaea there is a representative (Ta0583) even more similar to eukaryotic actins.
Actin occurs in the cell in two forms: as globular monomers called G-actin and as filamentous polymers called F-actin (i.e., filaments made up of multiple G-actin monomers). F actin may also be referred to as a microfilament. A molecule of adenosine triphosphate (ATP) or adenosine diphosphate (ADP) is attached to each actin strand, in turn associated with a Mg2+ cation. Of the different possible combinations between the forms of actin and the nucleotide triphosphate, actin G-ATP and actin F-ADP predominate in the cell.
G actin
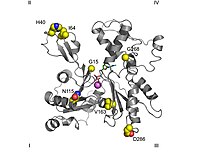
Regarding its molecular structure, G-actin has a globular appearance under the scanning electron microscope; however, by means of X-ray crystallography it can be seen that it is composed of two lobes separated by a slit; the structure forms the ATPase fold, an enzymatic catalysis center capable of binding ATP and Mg2+ and hydrolyzing the former to ADP plus phosphate. This fold is a conserved structural motif that is also present in other proteins that interact with nucleotide triphosphates such as hexokinase (an energy metabolism enzyme) or the Hsp70 proteins (a family of proteins that contribute to other proteins having functional structures). G-actin is only functional when it has either ADP or ATP in its cleft; however, in the cell, the ATP-bound state predominates when actin is free.

The actin crystallized by Kabsch, which is the most widely used as a model in structural studies, since it was the first to be purified, comes from rabbit skeletal muscle. It has dimensions of about 67 × 40 × 37 Å, a molecular mass of 41785 Da, and an estimated isoelectric point of 4.8. Its net charge at pH= 7 is -7.
- Primary structure
The complete amino acid sequence of this type of actin was determined by Elzinga et al. in 1973, and refined in subsequent works by the same author. Contains 374 amino acid residues. Its N-terminus is very acid. It begins with an acetylated aspartate in its amino group, while its C-terminus is basic, formed by a phenylalanine preceded by a cysteine of some functional importance. Both ends are located in a very close position within subdomain I. Regarding abnormal amino acids, it is worth noting an Nτ-methylhistidine in position 73.
- Tertiary structure-domains
It is made up of two domains known as large and small, separated by a cleft in the center of which is the ATP-ADP+Pi binding site. Below this there is a shallower notch called a "groove". When found natively, despite their name, both are comparable in size.
In topological studies, by convention, the protein is oriented so that the larger domain is on the left, while the smaller domain is on the right. At this position, the small domain is further divided into subdomain I (lower position, residues 1-32, 70-144, and 338-374) and subdomain II (upper position, residues 33-69). The major domain is also split into two others, subdomain III (lower, residues 145-180 and 270-337) and subdomain IV (upper, residues 181-269). The exposed area of subdomains I and III is called the "barbed" end, while that of subdomains II and IV is called the "arrowhead" end. This denomination refers to the fact that, due to the small mass of subdomain 2, actin acquires polarity, which will be discussed later when discussing assembly dynamics. Some authors name the subdomains as Ia, Ib, IIa and IIb, respectively.
- Other outstanding structures
- The most outstanding supersecundary structure is a five-chain β-ray that consists of a β-meandro and a β-α β dextrogira unit. It is present in both domains. This suggests that the protein emerged from gene duplication.
- The binding place to the nucleotid adenosin is between two structures in the form of β fork belonging to domains 1 and 3. The wastes involved are Asp11-Lys18 and Asp154-His161 respectively.
- Right below the nucleotide is the binding place to the divalent cathion, which in vivo is most likely the Mg2+ or the Ca2+ while in vitro it is formed by a burning structure in which the Lys18 and two oxygens of the α and β phosphates of the nucleotide contribute. This calcium is coordinated with six water molecules retained by the amino acids Asp11, Asp154, and Gln137. Along with the nucleotide forms a complex that restricts the movements of a region called hinge or hinge, located between residues 137 and 144, thus maintaining the native form of the protein, to the point that its withdrawal denaturalizes the actin monomer. This region is also important because it determines the "open" or "closed" conformations of the protein cleft.
- With almost all probability there are at least three other centers with less affinity (intermedia) and others with low affinity for divalent cations. It has been speculated about the role of these centers in the polymerization of the actin acting in the activation stage.
- In subdomain 2 there is a structure, called loop-D or D-loop because it joins DNAasa I, located between the His40 and Gly48 residues that appears as a disordered element in most crystals and as a β sheet when it is forming complex with DNAasa I. According to Domínguez et al., the key event of polymerization would be the propagation of a conformational change from the binding center to the nucleotide to this domain, which would become a loop to a propeller. This theory seems to be refuted by other works.
F-Actin
A classic description states that F-actin has a filamentous structure interpretable as a left-handed single-stranded helix with a turn of 166° and increment of 27.5 Å or as a right-handed double-stranded helix with half a thread pitch of 350-380 Å, each actin being surrounded by four others. The symmetry of the actin polymer, which is about 2.17 subunits per helix turn, is incompatible with the formation of crystals, which is only possible when there are exactly 2, 3, 4 or 6 subunits per round. Therefore, models must be made interpreting data from techniques that overcome these drawbacks, such as electron microscopy, cryoelectron microscopy, dimer crystals in different positions or X-ray diffraction. It is necessary to specify that speaking of a "structure" does not is correct for something as dynamic as an actin filament. In reality, one should speak of different structural states, among which the most constant data is the increase of 27.5 Å, while the rotation of the subunits shows considerable variability, being normal to observe displacements of up to 10% of their position. ideal. Some proteins, such as cofilin, appear to increase the angle of twist, but again this can be interpreted as stabilizing some normal "framework states" instead. These could be important in the polymerization process.
Regarding the radius of gyration or thickness of the filament, the measurements are more controversial: while the first models assigned a length of 25 Å, current X-ray diffraction data supported by cryoelectron microscopy agree at about 23.7 Å. These same studies have quite accurately determined the contact points between monomers. Some are established with units of the same chain, between the "bearded" end of one monomer and the "arrowhead" end of the next, while the monomers of adjacent chains make contact laterally through projections of subdomain 4, being the most The one formed by the C-terminus and a hydrophobic bond formed by three bodies in which residues 39-42, 201-203 and 286 intervene are important. To form part of a filament, according to this model, the monomers would be in a configuration called "flat", in which the subdomains rotate relative to each other, and also appears to be found in the bacterial MreB actin homologue.
Since all the subunits of a microfilament point towards the same end, the polymer is said to have polarity in its structure. This fact gives rise to a convention: the end that has an actin subunit is named exposing the place where it binds ATP to the medium as "end (−)" while at the opposite end, in which the cleft is directed to another adjacent monomer, is the "(+)" end. ». This method consists of the addition of S1 elements of myosin in tissues fixed with tannic acid; This myosin binds the actin monomers in a polar manner, giving rise to an arrow-like configuration with feathers along its entire shaft, where the shaft would correspond to actin and the feathers to myosin. Thus, the end of the microfilament with no protruding myosin is interpreted as the arrowhead, while the opposite end is called the barb.
In muscle, the helical F-actin filament also contains a molecule of tropomyosin, a 40-nanometer-long protein that wraps around the F-actin helix. During the cell's resting state, tropomyosin coats active sites on actin so that the actin-myosin interaction that produces muscle contraction is not achieved. Attached along the tropomyosin strand are other protein molecules, the troponins, complexes of three polymers: troponin I, troponin T, and troponin C.
Folding
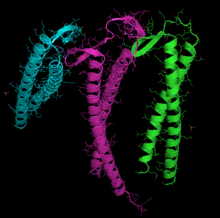
Actin can spontaneously acquire a large part of its tertiary structure. However, it displays a very special and almost unique behavior in the way that it acquires its fully functional form from its newly synthesized native form. The reason for such a special route could be the need to avoid the presence of misfolded actin monomers, which would be toxic, since they could act as inappropriate polymerization terminators. In any case, it is key for the stability of the cytoskeleton, and not only this, but it could be an essential process for the coordination of the cell cycle.
For this, it necessarily uses a type of group II cytosolic chaperonin (protein that helps others to fold), the CCT, formed by a double ring of eight different subunits (heteroctameric) that is distinguished from the other molecular chaperones, and especially its counterpart in archaea GroEL in that it does not require a co-chaperone that acts as a cover over the central catalytic cavity. It accepts substrates by binding to them through specific domains, for which reason it was initially thought to be exclusive of actin and tubulin, although immunoprecipitation has now shown that it interacts at least with a large number of polypeptides, possibly as substrates. It works through ATP-dependent conformational changes, sometimes requiring several rounds of release and catalysis to complete its work.
For their correct folding, actin and tubulin also specifically require the assistance of another protein, prefoldin, a heterohexameric complex (formed by six different subunits), and so specifically that they have even coevolved. In the case of actin, it immediately binds to it while it is still being translated, approximately when it is 145 amino acids long, which is the corresponding N-terminal domain.
Different recognition subunits are used for actin and tubulin, although they overlap. In the case of actin, it is probably the PFD3 and PFD4 subunits that bind to actin in two places, I, between residues 60-79, and II, between residues 170-198. Actin is recognized, loaded, and delivered to the CCT in an open conformation by the inner end of the "tentacles" of the prefoldin (see image and footnote). Contact at the time of delivery is so brief that a ternary complex is not formed, releasing the prefoldin immediately.
Subsequently, the cytosolic chaperonin (CCT) folds the actin sequentially, forming junctions with the subunits, instead of simply enclosing it in its cavity. To do this, it has specific recognition zones in its apical β domain. The first stage of folding would consist of the recognition of residues 245-249. Subsequently, other determinants would establish contact. Both actin and tubulin bind to the CCT in open conformations in the absence of ATP. In the case of actin, in each conformational change it binds to two subunits, unlike tubulin, which binds to four. Actin has specific binding sequences, interacting with the CCTδ and β subunits or with CCTδ and CCTε. After the binding of AMP-PNP to the CCT, the substrates move through the chaperonin cavity. It also seems that in the case of actin, the CAP protein is required as a possible cofactor in the final stages of actin folding.
The exact regulation of this process is not yet known, but it is known that the PhLP3 protein (phosducin-like protein) regulates its activity by inhibiting it, through the formation of a ternary complex.
ATPase catalytic mechanism
Actin is an ATPase, that is, an enzyme that hydrolyzes ATP. This set of enzymes is characterized by acting extremely slowly. It is known that this ATPase is "activated", or what is the same, its rate increases about 40,000 times when the actin is part of a filament. A reference value for this rate of hydrolysis under certain ideal conditions would be 0.3 s-1. Subsequently, Pi would remain bound to actin for a long time along with ADP, releasing near the end of the filament.
To this day, the specific molecular details of the catalytic mechanism are not known. Although there is much controversy about this, it seems clear that a "closed" conformation is required for ATP hydrolysis, and is believed to bring the residues involved the right distance. One of the key residues would be Glu137, located in subdomain 1 Its function would be to anchor the water molecule that produces a nucleophilic attack to the γ-phosphate bond of ATP, while the nucleotide binds strongly to subdomains 3 and 4. The slowness of the catalytic process is due to the great distance and biased position of this water molecule with respect to its reactant. Most likely, the conformational change produced by domain rotation between the G and F forms of actin brings Glu137 closer, allowing its hydrolysis. According to this model, polymerization and ATPase function would initially be uncoupled.
Assembly dynamics
F-actin combines the qualities of being resilient and dynamic. Unlike other polymers, such as DNA, which hold their constituent elements together by covalent bonds, in actin filaments the monomers are assembled by weaker, non-covalent bonds. This, which in principle weakens the structure, since it could be broken by thermal agitation, is solved by means of the lateral bonds with the neighboring monomers. At the same time, weak bonds maintain the advantage that the ends of the filament can easily release or incorporate monomers, so that they can rapidly remodel and change the cellular structure for which they are responsible in response to environmental stimuli. The latter and the biochemical mechanism by which it is effected is what is known as "assembly dynamics".
- Studies in vitro
Studies of the dynamics of microfilament subunit addition and loss have been performed in vitro (i.e., in the laboratory, outside of cellular systems) because the resulting actin polymer it gives rise to the same F-actin produced in vivo, where this process is controlled by a multitude of proteins to respond to cellular needs, so it would be very difficult to observe its basic conditions. In vitro, this event occurs sequentially: first, there is an "activation phase", in which the binding and exchange of divalent cations at specific sites on G-actin, bound to ATP, produce a change conformational, sometimes known as G-actin* or F-actin monomer, since it is more similar to the units that sit on the filament. This prepares it for the next "nucleation phase", in which G-actin gives give rise to small unstable fragments of F actin capable of polymerization. Initially dimers and trimers are formed in an unstable manner. When the number of these is large enough, the "elongation phase" occurs, in which the filament forms and grows rapidly by the reversible addition of new monomers to both ends. Finally, at "steady equilibrium", the G-actin monomers are exchanged at the ends of the microfilament without changing the total length of the polymer. In this last phase, the "critical concentration Cc" is defined as the ratio between the assembly constants and disassembly (it is, therefore, a dissociation constant), and represents the concentration of G-actin in which the dynamics of monomer addition and elimination does not produce a change in the length of the microfilament. Under the usual in vitro conditions, Cc is 0.1 μM, which means that higher values lead to polymerization and lower values lead to depolymerization.
- ATP hydrolysis paper
An important issue that was introduced in the previous section is the fact that, although actin hydrolyzes ATP, everything seems to indicate that this is not involved in assembly, since, on the one hand, hydrolysis occurs to a large extent inside the filament, and on the other, ADP can also polymerize. This raises the question of understanding what is the thermodynamically unfavorable process that requires such an enormous energy expenditure. The so-called «actin cycle», which links hydrolysis to polymerization, consists of the addition of Actin G-ATP monomers preferentially at the barbed end, creating a flow of monomers towards the arrowhead end in what is This is known as "threadmilling", where the monomers would be in the form of Actin F-ADP and would be released, subsequently exchanging this ADP for ATP and thus closing the cycle.
Shortly after addition, ATP hydrolysis occurs relatively quickly. There are two hypotheses about how it occurs: stochastic, in which hydrolysis would occur randomly, influenced to some extent by neighboring molecules, and vectorial, in which it would only occur at the limit with other molecules that have already hydrolyzed their own. ATP. In any case, the resulting Pi is not released, but remains non-covalently bound to ADP actin for a while, thus there would be three species of actin in a filament: ATP-Actin, ADP+ Pi-Actin and ADP-Actin. The content of a filament in each of these species depends on its length and state: at the beginning of elongation, the filament has a roughly equivalent composition of monomers with ATP and ADP+Pi and a small amount next to the (−) end of Actin ADP. As steady state is reached, the situation is reversed, with most of the filament containing ADP and the (+) end virtually only ADP+Pi, with ATP reduced to the extreme.
If we compare pure actin-ADP filaments with those that incorporate ATP, in the former the critical constants are similar at both ends, while in the other two nucleotides the Cc is different: At the (+) end it is Cc+=0.1 μM, while at the (−) end it is Cc−=0.8 μM, thus give the following situations:
- For G-ATP activity concentrations lower than Cc+ there is no elongation of the filament.
- For G-ATP activity concentrations less than Cc−but older than Cc+ elongation is given at the end (+).
- For G-ATP-actin concentrations greater than Cc− microfilament grows on both ends.
Therefore, it can be deduced that the energy of hydrolysis is used to create a true “steady state”, that is, of a flow instead of a simple equilibrium, which endows with dynamism, polarity and pulling force to the filament, which justifies the cost of gaining essential biological functions. In addition, the configuration of the different types of monomers is detected by the actin-binding proteins that control this dynamism, as will be seen in the next section.
There seems to be an exception in the typical way microfilaments are attached by braiding or threadmilling in the stereocilia. In this case, the control of the size of the structure would be entirely apical and in some way controlled by gene expression, that is, by the total amount of protein monomer synthesized at a given time.
Associated proteins
In vivo, the actin cytoskeleton is not composed exclusively of actin, but requires other proteins for its generation, permanence, and function; These are called actin binding proteins (ABPs, actin binding proteins) and are involved in its polymerization and depolymerization, stability, its organization into bundles or networks, its fragmentation and destruction. The diversity of these proteins is such that actin is considered to be the protein that participates in the largest number of known protein-protein interactions. For example, there are elements that sequester G-actin, preventing its incorporation into the microfilaments. In the same way, there are proteins that stimulate their polymerization or that provide complexity to the networks in synthesis.
- Timosine β4 is a 5 kDa protein capable of joining the G-ATP actin in a 1:1 stokyometry; this means that a β4 thymosine unit joins another G actin, in this proportion. Its role is to prevent the incorporation of monomers into the growing polymer.
- Prophylline, a 15 kDa cytolic protein that also binds in 1:1 schiometry to the G-ATP actin monomers, but its function is different: facilitates the exchange of ATP nucleotides by ADP. In addition, it is involved in other cell functions, such as the union of Pro repetitions in other proteins or lipids that act as second messengers.

Other actin-binding proteins regulate the length of microfilaments by cutting them, giving rise to new active ends for polymerization. That is, if a microfilament, which has two ends to which monomers can be attached or dissociated, is cut twice, three new microfilaments with six ends result; the new situation favors the dynamics of assembly and disassembly. Among these proteins, gelsolin and cofilin stand out. It should be noted that they first carry out the cut by means of changes in the conformation of the actin monomer to which they are attached in the polymer, remaining after covering the new (+) end generated, which prevents the addition or exchange of new G-actin subunits and, since the (-) ends remain uncoated, they favor the depolymerization of the filaments.
Other types of actin-binding proteins coat the ends of F-actin in order to stabilize them, without the ability to break them. Examples of these proteins are CapZ (which joins the ends (+) according to the levels of Ca2+/calmodulin in the cell, levels that depend on external and internal signals from the cell and that intervene in the regulation of its biological functions) or the tropomodulin (which joins the ends (-)). Tropomodulin is essential as a stabilizer of F-actin present in the myofibrils of muscle sarcomeres, structures characterized by their great stability.
The Arp2/3 complex is widely distributed in all eukaryotic organisms. It is composed of seven subunits, some of which have a topology clearly related to their biological function: two of its subunits, called "ARP2" and " ARP3», have a structure very similar to the actin monomers themselves. This homology allows both units to behave as nucleating agents for the polymerization of G-actin to F-actin monomers. In addition, this complex is necessary to establish dendritic structures and in anastomoses (that is, bifurcated or in a network), therefore, more complex, F-actin.
Chemical inhibitors
There are several toxins that interfere with actin dynamics, both depolymerizing them (latrunculin and cytochalasin D) and stabilizing them (phalloidin):
- Latrunculin, a toxin produced by perifers, joins the G actin by preventing its joining the microfilaments.
- The cytocalasine D, an alkaloid produced by fungi, joins the end (+) of the actin F preventing the addition of new monomers. Effects of cytocalasine D have been described, middle by the disruption of the dynamics of actins, in the activity of p53 (in animals) or in gravitropic responses (in plants).
- Faloidine, a toxin isolated from the fungus Amanita phalloides, it joins the existing interface between the adjoining actin monomers of the actin polymer F, which prevents the depolimerization of that.
Functions and location
Actin as a protein is found both in the cytoplasm and in the cell nucleus. This localization is regulated by the signal transduction pathways that integrate the stimuli that the cell receives and that allows the restructuring of actin networks in response to those. In Dictyostelium, the intervention of the phosphoinositide pathway mediated by phospholipase D has been reported. Actin filaments are especially abundant and stable in muscle fibers. Within the sarcomere (the morphological and physiological unit of muscle fibers) actin is arranged in bands I and A; in the latter, it occurs together with myosin.
Cytoskeleton
Microfilaments are involved in the movement of all mobile cells, including non-muscle cells, as drugs that disrupt F-actin (such as cytochalasins) have been reported to affect the activity of these cells. As a protein, actin accounts for 2% of the total protein in hepatocytes, 10% in fibroblasts, 15% in amoebas, and up to 50-80% in activated platelets. There are different groups of actin, with slightly different structure and function. different. Thus, α-actin is exclusive to muscle fibers, and that present in other cells is usually of the β and γ type. In addition, actin of types other than α usually has a high turnover rate, which means that most of it does not form part of permanent structures. Thus, microfilaments in non-muscle cells appear in two ways:
- Microfilament networks. Under the plasma membrane it is common in animal cells the appearance of a cell cortex populated by a multitude of microfilaments that excludes the presence of orgánulos. This network is related to abundant cell receptors that transmit signals from the outside of the cell.
- You make microfilaments. These microfilaments, arranged in networks, are of greater length and, in association with contactial proteins such as non-muscular myosine, intervene in the displacement of substances at the intracellular level.
Yeasts
In yeast, the actin cytoskeleton is key during the processes of endocytosis, cytokinesis, determination of cell polarity and during morphogenesis. These facts, in addition to depending on actin, implicate 20 or 30 associated proteins, highly conserved in evolution, as well as a multitude of signaling molecules; these elements allow, in combination, a spatially and temporally modulated assembly that defines cell biology in response to internal and external stimuli.
Yeasts have three main types of elements that are the product of actin association: patches, cables, and rings that, despite being detected for long periods of time, are subjected to a dynamic equilibrium due to continuous polymerization and depolymerization. As accessory proteins, they have a 16 kDa cofilin/ADF (encoded by a single gene, called COF1); Aip1, a cofilin cofactor that favors the disassembly of microfilaments; Srv2/CAP, a regulator of protein dynamics related to adenylyl cyclases; a profilin of approximately 14 kDa that associates with actin monomers; and twinfilin, a 40 kDa protein involved in the organization of patch-like structures.
Plants
Plant genomics studies have revealed the existence of protein isovariants within the actin gene family; Within Arabidopsis thaliana, a dicot used as a model organism, there are at least ten types of actins, nine from α tubulins, six from β tubulins, six from profilins and dozens of myosins. Such diversity is explained according to the evolutionary need to have slightly different variants in their pattern of temporal and spatial expression; however, most of them are jointly expressed in the tissues analyzed. The network of actin networks is distributed throughout the cytoplasm of cells cultured in vitro, with a reinforcement around the nucleus that is connected, by means of rays, to the cell cortex; This framework is highly dynamic, with a continuous polymerization and depolymerization.
Although plant cells generally have a wall that defines their morphology and prevents their movement, their microfilaments generate the necessary forces for various cellular activities, for example, the cytoplasmic currents generated by microfilaments and myosins. In addition, actin is involved in organelle movement and cell morphogenesis, processes that include cell division, elongation, and differentiation.
Regarding the proteins associated with the actin cytoskeleton present in plants, it is worth mentioning: villin, a protein belonging to the gelsolin/severin family, capable of cutting microfilaments and binding actin monomers in the presence of the calcium cation; fimbrin, an element capable of recognizing and binding actin monomers and involved in the formation of frameworks (through a regulation different from that of animal cells and yeasts); formins, proteins capable of acting as a nucleating agent for polymerization to F actin; myosin, a typical eukaryotic molecular motor that, in Arabidopsis thaliana, is encoded by 17 genes classified into two different classes; CHUP1, capable of binding actin and involved in the spatial distribution of chloroplasts in the cell; KAM1/MUR3, a protein that defines the morphology of the Golgi complex as well as the xyloglucan composition of the cell wall; NtWLIM1, a protein that facilitates the appearance of coiled actin structures; and ERD10, which participates in the association between organelles delimited by membranes and microfilaments and which seems to play a particularly relevant role in the presence of stress.
Muscle contraction
In muscle, the helical F-actin filament also contains a molecule of tropomyosin, a 40-nanometer-long protein that wraps around the F-actin helix. During the cell's resting state, tropomyosin coats the actin active sites so that actin-myosin interaction is not achieved (this interaction gives rise to a slippage between the two which, by coordination of many copies of these elements arranged in the muscles, produces their contraction). Attached along the tropomyosin strand are other protein molecules, the troponins, complexes of three polymers: troponin I, troponin T, and troponin C. The modulatory function of tropomyosin depends on its interaction with troponin in the presence of carbon ions. Ca2+.
Actin, along with myosin, is involved in muscle contraction and relaxation, the two constituting about 90% of muscle proteins. The overall process is triggered by an external signal, typically an action potential excitatory muscle that houses specialized cells rich in actin and myosin filaments within it. The contraction-relaxation cycle responds to the following steps:
- Deolarization of the sarcolema and transmission of the potential of action through T lobes.
- Opening of Ca2+ channels of the sarcolasmic reticle.
- Increased Cytolic Concentration of Ca2+ and interaction of these cations with the troponin causing a modification in its conformation, which in turn alters the structure of the tropomysine, which covers the active site of the actin, allowing the establishment of the cross links miosin-actine (the latter present as thin filaments).
- Movement of myosin heads over thin filaments, both independently and dependent on ATP. This latest mechanism, mediated by the ATPasa activity of the heads of myosin, causes the movement of the actin filaments to the Z disk.
- Capture of the Ca2+ on the part of the sarcoplasmic reticle, which causes a new conformational change in the thropomysine that inhibits the actin-myosin interaction.
Other biological processes
The classic study of the function of actin limits it to the maintenance of the cytoskeleton and, therefore, to the organization and movement of the organelles and determination of the cell shape. However, the role of actin is much more extensive in eukaryotic cell physiology; Furthermore, similar elements exist in prokaryotes.
- Citocinesis. In animal and yeast cells, cell division usually involves the separation of the stem cell into two daughter cells by constricting its equatorial area. In this process an actin, myosin, and α actininin ring is involved. In the fission yeast Schizosaccharomyces pombe, the actin is actively assembled in the contráctil ring with the participation of Arp3, the formina Cdc12, profilina and WASp, although they also involve preformed microfilaments. Once the ring is formed, the structure remains in a continuous assembly/disassembled which, with the help of the Arp2/3 complex and the forminas, becomes a central process of cytokinesis. The contractable ring set, microtubules of the acromatic spindle and the peripheral dense material is called "Fleming body" or "intermediate body".
- Apoptosis. During programmed cell death, the family of proteases called ICE/ced-3 (of the family of interleuquina-1β converters) degrade in vivo the actine in two fragments of 15 kDa and 31 kDa, which is one of the mechanisms of destruction of the cell viability on which apoptosis is based. This destruction has also been cited through proteasa calpain; so much so, that the use of calpain inhibitors decreases the proteolysis of the actin and, even, the degradation of DNA (other characteristic elements of apoptosis). On the other hand, the induction of the apoptosis process through stress passes through the reorganization of the actin cytoskeleton (which also implies its polymerization), giving rise to the structures called stress fibers; this fact is signaled through the pathway of kinase MAPs.
- Cellular Accession and Development. Cell adhesion is a character of multicellular organisms that sustains the ability to specialize in the tissue and, therefore, the increase in the complexity of those. The cell connections of the epitheliums use the actin cytoskeleton, within each cell, and cadherins, as extracellular elements, with a connection between both mediated by catheins. The disruption of the dynamics of actins impacts on the development of organisms; in fact, actin is such a crucial element that, generally, redundant gene systems are available. For example, the specimens Dictyostelium to those who had been deprived of the α actinine gene or the gelifying factor did not show an abnormal phenotype possibly because one of the proteins could perform the function of the other; instead, in the double mutants, lacking both, the development was altered.
- Modulation of gene expression. The status of actin polymerization influences the pattern of gene expression. In 1997, in works using Schwann cells, it was detected that depolimerization mediated by cytocalasine D caused a pattern of peculiar expression of the genes involved in the myelinization of this type of nerve cell. As for unicellular organisms, in some of its vital phases it has been shown that Actine F also modifies transcribing in the fungus Candida albicans. In addition, proteins similar to actin play a regulatory role during spermatogenesis in the mouse and, in yeasts, a role of proteins similar to actin in epigenetic modulation has been proposed. In fact, the actine is able, together with a type of nuclear myosine to interact with polymerase RNAs and other enzymes of the transcriptional machinery, to act as a transcription initiator.
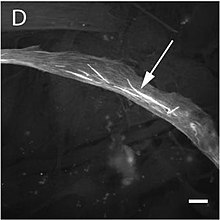
- Stereoly dynamics. Some types of cells develop on their surface a thin filiform evaginations with mecansensorial function called stereocilios. For example, these orgies are those involved in the sense of the ear in the organ of Corti. As a main feature, these structures have a length that can be modified. As for their molecular architecture, the stereos have a paracrystalline nucleus of actin in dynamic balance with the monomers present in the adjacent cytosol. Throughout this nucleus are available myosins of the types VI and VIIa, while the fifteenth myosine is at its ends and in amounts proportional to the length of the stereoly.
Molecular pathology
In most mammals there are six different actin genes. Two of them are related to the cytoskeleton (ACTB and ACTG1) while the remaining four are related to skeletal muscle (ACTA1), muscle smooth muscle (ACTA2), enteric smooth muscle (ACTG2) and with cardiac muscle (ACTC1). Mutations affecting these genes were unknown until 1998, and have been shown to cause myopathies, variations in cardiac size and function, and deafness. Likewise, the actin of the cytoskeleton is involved in the mechanism of pathogenicity of multiple infectious agents, including HIV. The vast majority of mutations affecting actin are point-type and have a dominant effect, except for at least six nemaline myopathy mutations. This is because in many cases the mutant variety of the actin monomer acts as "capping", that is, as a terminator for the elongation of the F actin.
Related to ACTA1
ACTA1 is the gene that encodes the α isoform of human actin present mainly in skeletal muscle, although it is also expressed in cardiac muscle and the thyroid gland. Its sequence consists of seven exons, which produce five known transcripts. As of 2006, the ENMC (European Neuromuscular Center) had published 116 mutations associated with pathologies, known as actinopathies. Most of them consist of point substitutions of amino acids, which in many cases can be associated with the phenotype that determines the severity and course of the condition.
They manifest by altering the structure and function of skeletal muscle, producing three forms of myopathy: type 3 nemaline myopathy, congenital myopathy with excess microfilaments (CM) and congenital myopathy with fiber type disproportionate (CFTDM). Mutations that produce myopathy with cores (areas devoid of oxidative activity) have also been detected. Although their phenotypes are similar, in addition to typical nemaline myopathy and intranuclear rod myopathy, some specialists distinguish a type of myopathy, actinic call of nemaline myopathy. In the first, actin aggregates accumulate instead of the typical rods. It is important to note that a patient may show more than one of these phenotypes on biopsy. The most common symptoms are typical facial morphology (myopathic facies), muscle weakness and delayed motor development, and respiratory difficulties. The course, severity, and age of onset are highly variable, and overlapping forms of myopathy are found. In nemaline myopathy, non-pathognomonic structures appear in various locations of type 1 muscle fibers known as "nemaline rods", with a composition similar to the z-discs of the sarcomere.
The pathogenesis is highly varied. Many mutations occur in the actin cleavage zone, close to the nucleotide binding site, while others occur in domain 2, or in the areas of interaction with associated proteins, which explains the great variety of aggregates. that are formed in these cases, such as nemaline bodies, intranuclear bodies, or zebra bodies. In nemaline myopathy, there are changes in the folding and aggregation properties of actin, and also in the expression of other associated proteins. In some variants in which intranuclear bodies are found, the folding change masks the nuclear export signal, so that aggregation of the mutant form of actin occurs in the cell nucleus. In contrast, it appears that in the mutations of ACTA1 that give rise to CFTDM, sarcomeric function is affected more than the structure itself. Recent work tries to clarify the apparent paradox that there is no clear correlation between rod abundance and weakness muscular. It seems that some particular mutations are capable of inducing a higher rate of apoptosis in type II muscle fibers.
Smooth muscle
There are two isoforms that encode smooth muscle actins:
ACTG2 encodes the longest isoform of actin, with nine exons, one of them, the one located at the 5' end, which is not translated. It is a γ actin that It is expressed in enteric smooth muscle. No mutations corresponding to pathologies have been found in this gene, although microarrays have shown that it is the protein that, by far, increases its expression the most in cases of resistance to chemotherapy with cisplatin.
ACTA2 encodes an α actin located in smooth muscle, and also in vascular smooth muscle. It has been seen that a mutation, MYH11, could be responsible for at least 14% of the cases of hereditary thoracic aortic aneurysms, specifically type 6, since the mutated variant produces a bad assembly of the filaments and a reduction of the contraction capacity of vascular smooth muscle. Medial aortic degeneration, with areas of disorganization and hyperplasia, and stenosis of the aortic vasa vasorum are seen in these individuals. The number of conditions in which this gene might be involved is increasing. It has been linked to Moyamoya disease, and it appears that some heterozygous mutations might confer a predisposition to many vascular pathologies, such as thoracic aortic aneurysm and ischemic heart disease. α Smooth muscle actin is also an interesting marker for evaluate the progression of liver cirrhosis.
Heart muscle
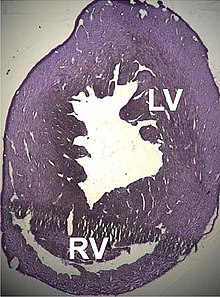
ACTC1 is the gene that encodes the α-actin isoform present in cardiac muscle. It was first sequenced by Hamada et al. in 1982, and it was observed that it was interrupted by five introns. It was the first gene of the six where alleles involved in pathological processes were found.
Several structural disorders leading to cardiac dysfunction associated with point mutations in this gene have been described, such as dilated cardiomyopathy type 1R and hypertrophic cardiomyopathy type 11. Recently, it has been seen that some atrial septal defects could also be related.
In the case of dilated cardiomyopathy, two cases have been studied in which both produce a substitution in highly conserved amino acids belonging to the domains that bind to the Z disks and interspersed, all of which leads to the hypothesis that the dilation is produced by a defect in the transmission of the contractile force in the myocytes.
Alterations of ACTC1 are responsible for less than 5% of hypertrophic cardiomyopathies. Several point mutations have also been demonstrated:
- E101K Mutation: net load changes and weak electrostatic link formation in the union position of the actuary.
- P166A: zone of interaction between actin monomers.
- A333P: Actina-miosine interaction zone.
The pathogenesis seems to obey a compensatory mechanism: the mutant proteins would act as "toxic" with a dominant effect, decreasing the contraction capacity with abnormal mechanical performance, so that hypertrophy, which is usually late, would be the consequence of a normal response of the heart muscle to stress.
Recently, ACTC1 mutations have been found to be implicated in two other pathological processes: childhood idiopathic restrictive cardiomyopathy, and non-compact left ventricular myocardium.
Of cytoplasmic actins
ACTB is a very complex locus. There are many pseudogenes distributed throughout the genome, and their sequence contains six exons that can give rise to up to 21 different transcripts by alternative splicing, known as β-actins. Consistent with this complexity, its products also have locations and are part of very different processes (cytoskeleton, NuA4 histone-acyltransferase complex, cell nucleus) and for this reason it has also been associated with the mechanism of a large number of pathological processes (carcinomas, juvenile dystonia, mechanisms of infections, malformations in the nervous system and invasiveness of neoplasms, among others). A new form of actin has been found, kappa actin, which seems to replace β-actin in tumor processes.
So far, three pathological processes have been detected that are due to a direct alteration of the sequence of a gene:
- Hemangiopericytoma with translocation t(7;12)(p22;q13) is a rare condition, in which a translocation fusion of the gene occurs ACTB on GLI1 in chromosome 12.
- Youth debut dystony is a rare degenerative disease, with systemic involvement of the central nervous system, and especially of neocortical and thalamic areas, where you can see a type of eosinophilic inclusions in the form of a cane. Affected individuals have a phenotype with median malformations, sensory hearing loss and dystony. It is due to a punctual mutation that changes arginine amino acid in position 183 by a tryptophan. This alters the interaction of the actin with the ADF/cophylline system, which regulates the formation dynamics of the neuronal cytoskeleton.
- A dominant punctual mutation has been found that causes neutrophil dysfunction and recurrent infections. It seems that the mutation modifies the binding domain with profiline and other regulatory proteins. Affinity for profiline in this allele is very small.
ACTG1 is the locus encoding the cytosolic γ-actin protein responsible for the formation of microfilaments of the cytoskeleton. It contains 6 exons, giving rise to 22 different mRNAs, which produces 4 complete isoforms, possibly expressed in a tissue-dependent manner. It also has two alternative promoters. The translated sequences of this locus and the β actin locus have been shown to be more similar than expected, suggesting a common ancestral sequence that underwent duplication and gene conversion.
From a pathological point of view, it has been associated with processes such as amyloidosis, retinitis pigmentosa, infection mechanisms, kidney diseases and various congenital hearing losses.
Related to six autosomal-dominant point mutations in the sequence, we found various forms of hearing loss, especially sensorineural type 20/26. It appears that they specifically affect the stereocilia of the hair cells of the organ of Corti. β actin is the most abundant protein in human tissues, but not in hair cells, which would explain the location of the pathology. On the other hand, it seems that most of these mutations affect binding sites with other proteins, especially actomyosin. Some experiments suggest that the pathogenic mechanism of this type of deafness is due to the fact that the F actin of the mutants would be more sensitive than usual to cofilin.
On the other hand, although there is no record of any case, it is known that γ actin is also expressed in skeletal muscle, and although in very small amounts, animal models have shown that its absence could lead to myopathies.
Other pathological mechanisms
Some infectious agents use actin, especially cytoplasmic, in their life cycle. In bacteria there are basically two forms:
- Listeria monocytogenes, some species of Rickettsia, Shigella flexneri and other intracellular germs escape from fagocytic vacuolas by coating with a short capsule of actin filaments. In the case of L. monocytogenes and S. flexneri, they generate from them a trail in the form of "cometail" that allows their mobility. There are slight differences in the molecular mechanism of polymerization of the "cola in comet" depending on the bacteria species. Different speeds of displacement can be observed, for example, with a maximum for Listing and Shigella. Many experiments have tested this mechanism in vitro. These show that no miosin type motor protein is used, and it seems that the propulsion is acquired by the pressure exerted by the polymerization that takes place near the wall of the microorganism, which has previously been surrounded by ABP's own of the host cell, which in its minimum configuration would be the Arp2/3 complex, Ena-VASP protein, cofiline, a vinonant protein and promoters. Through these movements they form protrusions that reach the neighboring cells, infecting them in turn, so that the immune system can only combat infection through cellular immunity. The route of the movement could be due to the modification of the curvature and deramification of the filaments. Other species, such as Mycobacterium marinum and Burkholderia pseudomallei, they are also able to polymerize the cellular actin locally to facilitate its displacement by means of a mechanism that pivots on the Arp2/3 complex; moreover, the vaccine virus or Vaccinia virus It also uses elements of the actin cytoskeleton for its dissemination.
- Pseudomonas aeruginosa is able to form a protective biofilm that escapes from the defenses of the organism, especially neutrophils and antibiotics, using DNA and actin filaments of the host.
In addition to the example cited above, in the initial steps of internalization of some viruses, notably HIV, actin polymerization is stimulated, for example by inactivating cofilin.
In the invasion processes of cancer cells, actin-based protrusions play a role not yet determined.
Evolution
The eukaryotic cytoskeleton shows some highly similar components along the phylogenetic scale, especially actin and tubulin. For example, the protein encoded by the human ACTG2 gene has absolute equivalence with the orthologs present in rats and mice, although at the nucleotide level the identity decreases to 92%. However, it does there are greater differences with the equivalents in prokaryotes (FtsZ and MreB), which, in turn, present a sequence identity of between 40-50% between the different species of bacteria and archaea. Some authors suggest that the ancestral protein that gave rise to the basic eukaryotic actin model resembles the proteins of the bacterial cytoskeleton present today.
Some authors highlight that actin, tubulin and histones, a type of protein involved in the stabilization and regulation of DNA, present similarities in their ability to unite nucleotides and in their functioning based on the use of Brownian movement; moreover, they suggest that all of them could derive from a common ancestor. Therefore, evolutionary mechanisms diversified the ancestral protein into the variants present today, conserving, among others, actins as effective molecules to address ancient and essential biological processes, such as endocytosis.
Bacterial equivalents
Although bacteria do not have a cytoskeleton comparable in complexity to that of eukaryotes, proteins with high similarity to actin monomers and polymers have been described. The bacterial MreB protein polymerizes into thin, non-helical filaments and, rarely, into helical structures resembling F-actin. Furthermore, its crystal structure is very similar to G-actin (in terms of three-dimensional conformation), and there are even equivalences between MreB protofilaments and F actin. The bacterial cytoskeleton also has among its components the FtsZ proteins, similar to tubulin.
Bacteria therefore possess a cytoskeleton with actin-homologous elements (for example, MreB, ParM, and MamK), although the amino acid sequence of these proteins diverges from those present in animal cells. Nevertheless, MreB and ParM have a high structural similarity with eukaryotic actin. The highly dynamic microfilaments generated by aggregation of MreB and ParM are essential for cell viability and are involved in cell morphogenesis, genophore segregation, and cell polarity. ParM, a plasmid-encoded homologue of actin, is involved in the management of plasmid DNA.
Applications
The use of actin in science and technology laboratories derives from its participation as a rail of molecular motors such as myosin (either in the muscle or outside of it), and from its necessary presence for cell function. As for the symptoms, since some abnormal actin variants are related to the appearance of pathologies, their detection is a diagnostic criterion.
- Nanotechnology. Actine-miosine systems act as molecular motors that allow the transport of vesicles and orgánulos throughout the cytoplasm. There are experiments that take advantage of this dynamic capacity, even in vitro, that is, in acellular systems, so a nanotech application of the system has been postulated. The underlying idea is to use microfilaments as rails on which one or more motor proteins are moved by transporting a particular load; that is, to define a space circuit by which a particular load can be transported in a directed and more or less controlled manner. As for general applications, we talk about the directed transport of molecules to achieve their release in specific places, which would allow the assembly of nanostructures in a controlled way. These capabilities could be applied in research chips like lab on a chip, in mechanical nanocomponents and in mechanical energy nanotransformers in electric.
- Internal control in molecular biology techniques, such as western blot and the RCP in real time. Because the function of the actin is necessary for cell survival, it was postulated that its amount is so controlled at the level of cell production that it can be assumed that its transcription (i.e., the degree of expression of its genes) and translation, (which is the generation of protein) is practically constant, regardless of the experimental conditions. For this reason, in protein quantification studies (western blot) and transcribes (Real-Time PCRs) are usually performed in addition to the quantification of the gene of interest, that of a reference gene, such as the one mentioned actin. Dividing the amount of the gene of interest to that of the actine it is possible to obtain a relative amount comparable between different experiments, provided that the expression of the latter does not vary; it should be noted that the actin does not always present the desired stability in its expression.
- Clinic. Some alleles of the actin are cause of pathologies, so techniques have been developed for detection. In addition, the actin can be used as an indirect marker in surgical pathology: it is possible to use variations in location patterns in tissues as markers of neoplasm invasion, vasculitis and others. Also, due to its relationship with the muscle contraction apparatus, atrophy causes the decrease of its levels in the skeletal muscle, so it can be used as a marker of this phenomenon.
- Food technology. The determination of the quality of some processed foods, such as sausages, goes through the quantification of their meat content. Basically, a method based on the detection of 3-methylhystidine in hydrolysed of these products has been used, as it is a compound present in the actin and heavy chain of the F myosin (both majoritarian components of the muscle). The animal generation of the compound is due to the methylation of histidine residues present in both proteins.
Contenido relacionado
Brachypodium
Impatiens balsamina
Aira