Acid halide
An acid halide (or acyl halide) is a compound derived from an acid by replacing the hydroxyl group with a halogen.
If the acid is a carboxylic acid, the compound contains a -COX functional group. In them, carbon is attached to a radical or hydrogen atom (R), to an oxygen by a double bond and by a single bond (sigma) to a halogen (X).
The residue from removing the OH group is called an acyl group. Acid halides are named, then, by putting the name of the halogen before the name of the acyl moiety, which is named by replacing the ending "oic" from the acid from which it is derived by "yl" For example, the acyl moiety derived from acetic acid (CH3-CO-) is acetyl. The acid chloride derived from acetic acid will therefore be named acetyl chloride.
These compounds give nucleophilic substitution reactions very easily and are used in acylation reactions (such as Frieldel-Crafts).
The hydroxyl group of the sulfonic acid can also be replaced by a halogen, giving rise to a halosulfonic acid. For example, chlorosulfonic acid results from replacing a hydroxyl group with a chlorine atom.
Preparation
The most common laboratory methods for the preparation of acyl halides include the reaction of carboxylic acid with thionyl chloride (SOCl2), phosphorous pentachloride (PCl5 >) or oxalyl chloride ((COCl)2) to obtain the acyl chloride and with phosphorous tribromide for the acyl bromide.
Reactions
Acid halides give the typical nucleophilic substitution reactions of acid derivatives.
Hydrolysis (conversion to acids)
R− − COCl+H2OΔ Δ R− − COOH+HCl{displaystyle R-COCl+H_{2}Olongrightarrow R-COOH+HCl}
This reaction is a typical example of the type of acyl nucleophilic substitution reactions using the addition-elimination mechanism. The mechanism is as follows:
Alcoholysis (conversion to esters)
R− − COCl+R♫− − OHΔ Δ R− − COO− − R♫+HCl{displaystyle R-COCl+R'-OHlongrightarrow R-COO-R'+HCl}
Ammonilysis/Aminolysis (conversion to amides)
R− − COCl+2NH3Δ Δ R− − CONH2+NH4+Cl− − {displaystyle R-COCl+2NH_{3}longrightarrow R-CONH_{2}+NH_{4}{+}Cl^{-}
Primary and secondary amines and ammonia react with acyl chlorides to give an amide plus hydrogen chloride. The latter can be neutralized with a base or excess amine. In the mechanism we can see that in the last stage, the nitrogen loses a proton resulting in the amide, for this reason, tertiary amines do not form amides with acyl chlorides.
Conversion to acid anhydride
Its reaction with alkaline esters gives rise to anhydrides:
R− − COCl+R− − COO− − Na+Δ Δ (R− − CO)2O+Na+Cl− − {displaystyle R-COCl+R-COO^{-}Na^{+}longrightarrow (R-CO)_{2}O+Na^{+}Cl^{-}
Conversion to ketones
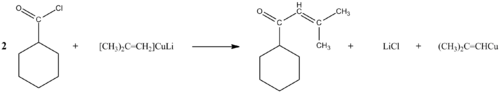
A. With Gilman's Reagent
By means of Gilman's reagents they give cetonas and organometallic haluros: R− − COCl+R2♫CuLiΔ Δ R− − CO− − R♫+Li+Cl− − +R♫Cu{displaystyle R-COCl+R'_{2}CuLilongrightarrow R-CO-R'+Li^{+Cl^{-}+R'Cu}
Organometallic compounds give ketones but however, some of them can continue to react with ketones to end up giving alcohols, to prevent this, diorganocuprates are used to prevent this instead of using RLi or RMgX which are less selective.
B. Friedel-Crafts acylation
Alkyl halides can be used to functionalize aryl groups via the Friedel-Crafts reaction:
R− − COCl+C6H6→AlCl3C6H5− − CO− − R+HCl{displaystyle R-COCl+C_{6}H_{6}{xrightarrow[{{{}{AlCl_{3}}}}C_{6}H_{5}-CO-R+HCl}
Conversion to tertiary alcohols
R− − COCl+2R♫− − MgIΔ Δ R− − COH− − R2♫{displaystyle R-COCl+2R'-MgIlongrightarrow R-COH-R'_{2}}
Conversion to aldehydes
R− − COCl+[chuckles](CH3)3− − CO]3AlLi− − HΔ Δ R− − CHO{displaystyle R-COCl+[(CH_{3})_{3}-CO]_{3Al}Li-Hlongrightarrow R-CHO}
Curtius Transposition
Curtius' transposition is a chemical reaction that allows a primary amine to be obtained by reacting a sodium acid halure. It forms an acile azide as an intermediary. The reaction occurs through a complex reaction mechanism, very similar to that of the Hofmann transposition, which includes the transfer of the alkylo group.

Contenido relacionado
Piepho–Krausz–Schatz model
Saponification
Germanium









![{displaystyle R-COCl+C_{6}H_{6}{xrightarrow[{}]{AlCl_{3}}}C_{6}H_{5}-CO-R+HCl}](https://wikimedia.org/api/rest_v1/media/math/render/svg/585b37b066b3fdbe7d1b68bd7f42cd3a88621287)

![{displaystyle R-COCl+[(CH_{3})_{3}-CO]_{3}AlLi-Hlongrightarrow R-CHO}](https://wikimedia.org/api/rest_v1/media/math/render/svg/200790ce6cb62da855703d53bd39dff35c340112)