Acetylsalicylic acid
acetylsalicylic acid or ASA (C9H8O4), popularly known as aspirin, a brand name that came into common use, is a drug of the salicylate family. It is used as a medicine to treat pain (analgesic), fever (antipyretic) and inflammation (anti-inflammatory), due to its non-selective inhibitory effect on cyclooxygenase.
It is also used to treat specific inflammations such as Kawasaki disease, pericarditis or rheumatic fever. Taking aspirin soon after a heart attack lowers the risk of death, and long-term use helps prevent heart attacks, strokes, and blood clots in people at high risk. It may lower the risk of heart attacks. certain types of cancer, especially colorectal cancer. In the treatment of pain or fever, its effects typically begin within thirty minutes. Acetylsalicylic acid is the non-steroidal anti-inflammatory drug (NSAID) par excellence and works similar to other NSAIDs, although it blocks the normal functioning of platelets (antiplatelet agent).
Common side effects include dyspepsia, and major side effects include peptic ulceration, stomach perforation, and worsening asthma. The risk of bleeding increases in the elderly, consumers of alcohol, other non-steroidal anti-inflammatory drugs or anticoagulants. Aspirin is not recommended in women who are in the last phase of pregnancy. In general, it is also not recommended in children with infections, due to the risk of Reye's syndrome, and in high doses it can cause tinnitus (ringing in the ears).
Salicylic acid, present in the leaves of the willow tree (Salix), has been used by mankind for at least 2,400 years. Acetylsalicylic acid was first synthesized by the chemist Frenchman Charles Frédéric Gerhardt in 1853, by combining sodium salicylate with acetyl chloride. In the second half of the 19th century other chemists described its chemical structure and devised more efficient methods for its synthesis. In 1897, Bayer scientists began studying aspirin as a possible less irritating replacement for common salicylate medications. Although prior to 1899, Bayer had called the drug "Aspirin" and marketed it under that brand name throughout the world, The company's rights to the brand were lost or sold in many countries. Its popularity grew during the first half of the XX century, which led to strong competition between different brands and products whose active ingredient it was acetylsalicylic acid.
Aspirin is one of the most widely used drugs in the world, with an estimated consumption of 40,000 tons per year, or in other words, between 50,000 and 120,000 million pills. It is on the list of essential medicines from the World Health Organization, where the basic medicines that every health system should have are classified. Aspirin is also available as a generic medicine and the wholesale cost in developed countries in 2014 was between 0.002 and $0.025 (USD) per dose. In the case of the United States, in 2015 a month of medication had an average cost of less than 25 dollars (USD).
History
The ancient Egyptians may have used the bark of the white willow (Latin for Salix alba) for medicinal purposes.
The Sumerians and Chinese used willow leaves as a pain reliever before 1000 B.C. C.
The first mention is found in the texts of Hippocrates (460-370 BC), father of Greek medicine, who used a concoction extracted from the leaves and bark of the willow Salix Latinum to relieve the pain and fever of his patients,
Also in some Amerindian culture (in the American continent) the bark of the white willow may have been used for medicinal purposes.
There is evidence that among the Hispano-Roman peoples there were some possible examples of the use and sacredness of the willow.
The medicinal effects of white willow continued to be mentioned by ancient authors such as the Roman polygraph and naturalist Pliny the Elder (23-79), the Greek physician and pharmacist Dioscorides (40-90) or the famous Greek physician Galen (130- 200).
During the Middle Ages, willow bark was boiled and given to people suffering from ailments to drink. However, this divine concoction was forgotten due to a law that restricted the barking and cutting of the leaves of this willow, since they were used in the basket making industry.
In the post-Renaissance era (1763) Edward Stone, Reverend of the Church of England, presented a report to Lord Macclesfield, who presided over the Royal Society, regarding these therapeutic properties of white willow bark, highlighting its antipyretic effect. Stone described in his work that he had administered the extract in the form of tea or beer to 50 febrile patients, relieving their symptoms. Further research led to the active ingredient in this plant, which scientists named salicillin, a precursor to salicylic acid and acetylsalicylic acid.
The active principle of willow bark was isolated in 1828 by Johann Buchner, professor of Pharmacy at the University of Munich, who reported that it was a bitter and yellowish substance, in the form of crystalline needles that he called salicin. However, two years earlier, the Italians Brugnatelli and Fontana isolated the same extract, but in a highly impure form, and failed to prove that the substance was responsible for the pharmacological effects of white willow bark. In 1829 a French pharmacist, Henri Leroux, improvised an extraction procedure from which he obtained 30 grams of salicin from 1.5 kg of bark. In 1838 Raffaele Piria (Italian chemist), working at the Sorbonne in Paris, managed to separate salicin into sugar and an aromatic component called salicylaldehyde. He converted the latter compound, by hydrolysis and oxidation, into colorless crystals which he named salicylic acid.
Acetylsalicylic acid was first synthesized by French chemist Charles Frédéric Gerhardt in 1853, wanting to improve the bitter taste and other side effects of salicylic acid such as irritation of the stomach lining, by combining sodium salicylate with acetyl chloride; and later as a salt by Hermann Kolbe in 1859. However, it was not until 1897 that the German pharmacist Felix Hoffmann, a Bayer laboratory researcher who, looking for an effective relief against the pain that his father suffered from chronic rheumatism treated with salicylic acid as well as important side effects, managed to synthesize acetylsalicylic acid with great purity. Its therapeutic properties as an analgesic and anti-inflammatory were described in 1899 by the German pharmacologist Heinrich Dreser, which allowed its commercialization.
Many years later, in 1949, Arthur Eichengrün, who was Hoffmann's direct boss, published an article claiming the discovery. It would be something truly amazing, for someone to claim credit for themselves 50 years later, when aspirin had already become a famous drug throughout the world for decades. In fact, this claim was ignored by scientific historians until 1999, when Walter Sneader's researcher at the University of Strathclyde (in Glasgow) once again postulated that it was Eichengrün who had the idea of synthesizing acetylsalicylic acid. In any case, the Bayer house, which would care little if the merits should fall to one or another of its employees, and which logically has all the documents that affect the case, refuted this hypothesis in a press release, but the controversy still open.
Aspirin was the trade name coined by Bayer laboratories for this substance, becoming the first drug in the group of NSAIDs (non-steroidal anti-inflammatory drugs). Later, in 1971, British pharmacologist John Robert Vane, then employed by the Royal College of Surgeons, London, was able to show that ASA suppresses the production of prostaglandins and thromboxanes, opening up the possibility of its use. in low doses as an antiplatelet agent, greatly expanding its commercial field and compensating for the fact that, currently, its use as the anti-inflammatory of choice has been displaced by other more effective and safe NSAIDs. In 1985 the Secretary of the United States Health Service, Margaret Heckler, announced that a daily dose of aspirin helped people who had suffered a myocardial infarction to prevent new attacks of coronary ischemia. During the First World War (1914-1919), the "aspirin" brand was expropriated in the winning countries, mainly the United Kingdom, the United States and France; in such a way that in these countries aspirin became the generic name of the substance.
Aspirin is now a registered drug in more than 70 countries around the world. Since its commercialization, more than three hundred and fifty billion tablets have been consumed and it is estimated that daily consumption is about one hundred million aspirins. Consequently, it is one of the most widely used drugs in the world, with an estimated consumption of more than 100 metric tons per day. Currently, 100% of the global production of acetylsalicylic acid manufactured by Bayer is carried out in Langreo, Spain, in a chemical plant of this multinational company. From there it is sent to different parts of the world where the tablets and different pharmaceutical forms in which the Aspirin is sold are prepared.
Description
Salicylic acid or salicylate, the metabolic product of aspirin, is a simple organic acid with a pKa of 3.0. Aspirin, on the other hand, has a pKa of 3.5 at 25 °C. Both aspirin and sodium salicylate are equally effective as anti-inflammatories, although aspirin tends to be more effective as a pain reliever.
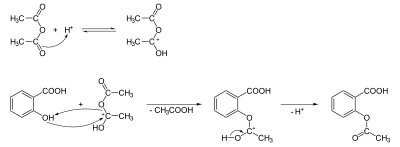
In the production of aspirin, oxygen is protonated to make a stronger electrophile.
The chemical reaction of the synthesis of aspirin is considered an esterification. Salicylic acid is treated with acetic anhydride, a compound derived from an acid, which causes the hydroxyl group of the salicylate to convert to an acetyl group (salicylate-OH → salicylate-OCOCH3). This process produces aspirin and acetic acid, which is considered a byproduct of the reaction. Acetic acid production is the reason why aspirin often smells like vinegar.
Small amounts of sulfuric acid and occasionally phosphoric acid are almost always used as a catalyst. The method is one of the most widely used reactions in chemistry laboratories at undergraduate universities.
Pharmacokinetics
Routes of administration (forms of use)
Acetylsalicylic acid is administered primarily orally, although it also exists for rectally, intramuscularly, and intravenously. Aspirin tablets for oral administration hydrolyze easily when exposed to water or humid air, so they should remain stored in their wrappers until the time of administration. Aspirin that has been hydrolyzed in this way gives off a vinegary odor (actually acetic acid) and should not be ingested. Aspirin also comes in chewable preparations for adults. Effervescent and flavored preparations are suitable for those who prefer liquid administration of the medication.
Severe stomach problems are more likely with non-enteric-coated aspirin.
Absorption
Aspirin has very poor solubility in low pH conditions ―such as inside the stomach―, a fact that can delay the absorption of large doses of the drug by 8-24 hours. All salicylates, including aspirin, are rapidly absorbed from the digestive tract at the level of the duodenum and small intestine, reaching the maximum concentration in the blood plasma after 1 to 2 hours. Because it is a weak acid, very little remains in the ionized form in the stomach after oral administration of salicylic acid. Due to its low solubility, aspirin is absorbed very slowly in cases of overdose, causing plasma concentrations to rise continuously for up to 24 hours after ingestion. Bioavailability is very high, although absorption tends to be affected by stomach content and pH.
Distribution
The binding of salicylate to plasma proteins is very high, greater than 99%, and of linear dynamics. Saturation of the binding sites on plasma proteins leads to a higher concentration of free salicylates, increasing the risk of toxicity. It has a wide tissue distribution, crossing the blood-brain and placental barriers. The serum half-life is approximately 15 minutes. The volume of distribution of salicylic acid in the body is 0.1-0.2 l/kg. States of acidosis tend to increase the volume of distribution because they facilitate the penetration of salicylates into tissues.
Metabolism
Aspirin is partially hydrolyzed to salicylic acid due to the first-pass effect in the liver (much of the acetylsalicylic acid does not reach the systemic circulation as it passes from the digestive system to the liver thanks to the portal vein). This hepatic metabolism is subject to saturation mechanisms, so when the threshold is exceeded, aspirin concentrations increase disproportionately in the body. It is also hydrolyzed to acetic acid and salicylate by esterases in tissues and blood.
Aspirin passes through the liver, and is then absorbed into the bloodstream, thus helping to soothe pain and general discomfort.
Pharmacodynamics
Mechanism of action
The biological mechanisms for the production of inflammation, pain or fever are very similar. They involve a series of substances that have a common end. Substances known as prostaglandins are generated in the area of the lesion. They could also be called "pain messengers". These substances inform the central nervous system of the attack and the biological mechanisms of inflammation, pain or fever are set in motion. In 1971 the British pharmacologist John Robert Vane demonstrated that acetylsalicylic acid acts by interrupting these mechanisms of production of prostaglandins and thromboxanes. Thus, thanks to the use of aspirin, normal body temperature is restored and pain is relieved. The ability of aspirin to suppress the production of prostaglandins and thromboxanes is due to the irreversible inactivation of cyclooxygenase (COX), an enzyme necessary for the synthesis of these proinflammatory molecules. The action of aspirin causes acetylation (ie, adds an acetyl group) at a serine residue in the COX active site.
Effects
Anti-inflammatory effects
Aspirin is a nonselective inhibitor of both isoforms of cyclooxygenase, but salicylate, the normal metabolic product of aspirin in the body, is less effective at inhibiting both isoforms. Salicylates that are not acetylated may have roles in oxygen radical scavenging. Aspirin irreversibly inhibits COX-1, modifies COX-2 enzymatic activity and inhibits platelet aggregation, but non-acetylated salicylate species do not. COX-2 usually produces prostanoids, most of which are pro-inflammatory. Modified by aspirin, COX-2 instead produces lipoxins, which tend to be anti-inflammatory. Newer NSAIDs (non-steroidal anti-inflammatory drugs) have been developed to inhibit COX-2 only to reduce the gastrointestinal side effects of COX-1 inhibition.
Aspirin also interferes with chemical mediators of the kallikrein-kinin system, thereby inhibiting granulocyte adherence to damaged vasculature, stabilizing lysosomes, thus preventing the release of inflammatory mediators, and inhibiting chemotaxis of polymorphonuclear leukocytes and macrophages.
Analgesic effects
Aspirin is most effective in reducing mild to moderate pain through its effects on inflammation and because it may be able to inhibit pain stimuli at the subcortical brain level. It is a weak organic acid that has both a carboxylic acid and phenol function, since it is also considered the orthophenol of benzoic acid (its name is orthophenomethyloic). It has anti-inflammatory characteristics but because it causes stomach irritations it is not applied as such but in the form of its derivatives, the best known being acetylsalicylic acid ("Aspirin") and methyl salicylate (the ester with alcohol methyl).
Antipyretic effects
Aspirin reduces fever, while taking it only slightly affects normal body temperature. The antipyretic effects of aspirin are probably mediated both by inhibition of COX in the central nervous system and by inhibition of interleukin-1, released by macrophages during episodes of inflammation.
Aspirin has been shown to disrupt oxidative phosphorylation in mitochondria of cartilage and liver by diffusing into the space between the two mitochondrial membranes and acting as a transporter for the protons required in the processes of cellular respiration. With the administration of high doses of aspirin, the appearance of fever is observed due to the heat released by the electron transport chain found in the inner membrane of mitochondria, contrary to the antipyretic action of aspirin at therapeutic doses. In addition, aspirin induces the formation of nitric oxide (NO) radicals in the body, which reduces the adhesion of leukocytes, one of the important steps in the immune response to infections, although there is still no conclusive evidence that aspirin be able to fight an infection.
Recently published data suggest that salicylic acid and other aspirin derivatives modulate their cell signaling actions via NF-κB, a complex of transcription factors that play important roles in many biological processes, including inflammation.
Antiplatelet effect
Low doses of aspirin, 81 mg daily, produce a slight prolongation of bleeding time, which doubles if aspirin administration is continued for a week. The change is due to the irreversible inhibition of COX in platelets, which is why it is maintained throughout their life (between 8 and 10 days). This anti-platelet aggregating property makes aspirin useful in reducing the incidence of heart attacks in some patients. Aspirin 40 mg per day is sufficient to inhibit an adequate proportion of thromboxane A2, without having an inhibitory effect on prostaglandin I2 synthesis, so higher doses will be required to have anti-inflammatory effects.
In 2008, a trial showed that aspirin does not reduce the risk of a first heart attack or stroke, but rather reduces the risk of a second event for those who have already had a heart attack or stroke. Women taking low-dose aspirin every other day lowers the risk of stroke, but it is not a treatment that can substantially alter the risk of heart attack or cardiovascular death. In general, for a patient who does not have heart disease, the risk of bleeding outweighs any benefit from aspirin.
Interactions
| Pharmacodynamic interactions of acetylsalicylic acid | |
| Fármaco | Interaction results. |
|---|---|
| • Nonsteroidal anti-inflammatory drugs (NSAIDs). | It can increase the risk of ulcers and gastrointestinal bleeding due to a synergistic effect. |
| • Corticoids | It can increase the risk of ulcers and gastrointestinal bleeding due to a synergistic effect. |
| • Diuretics | Joint administration may cause acute kidney failure, especially in dehydrated patients. In the event that acetylsalicylic acid and diuretic acid are administered simultaneously, it is necessary to ensure proper hydration of the patient and monitor the kidney function when starting the treatment. |
| • Selective Serotonin Reuptake Inhibitors (ISRS). | The risk of bleeding in general and high digestive bleeding in particular increases. |
| • Oral anticoagulants | The risk of bleeding increases, so it is not recommended. If it is impossible to avoid such an association, careful monitoring of INR is required. |
| • Trombolytics and antiplatelet antiplatelet | It increases the risk of bleeding. |
| They exercise a synergistic effect on reducing glomerular filtration, which can be exacerbated in case of renal function alteration. The administration of elderly or dehydrated patients may lead to acute kidney failure by direct action on glomerular filtration. In addition, they can reduce the antihypertensive effect, due to the inhibition of prostaglandins with vasodilating effect. |
| • β-blockers | Decrease in the antihypertensive effect due to inhibition of prostaglandins with vasodilating effect. |
| • Insulin and sulfonylureas | The hypoglycemic effect increases. |
| • Cyclosporine | It increases the nephrotoxicity of cyclosporin due to mid-effects of renal prostaglandins. |
| • Vancomicina | The risk of ototoxicity of vancomicine increases. |
| • Interferon α | Decrease the activity of the interferon-α. |
| • Alcohol | The risk of digestive bleeding increases. |
Pharmacokinetic interactions will cover practically the entire spectrum of possibilities in terms of the mechanism of production, although those of metabolic origin appear to be more interesting. In this sense, it appears to be independent of CYP3A4 and, like other NSAIDs, to be linked to CYP2C9. However, its abundant metabolism outside the liver means that its interactions at the level of cytochrome P450 are not essential. The most interesting are shown in the following table:
| Pharmacokinetic interactions of acetylsalicylic acid. | |
| Fármaco | Interaction results |
|---|---|
| • Metotrexate | They decrease the tubular secretion of metotrexate by increasing the plasma concentrations of it and therefore its toxicity. |
| • Lithium | They decrease lithium excretion, increasing blood lithium levels, which can reach toxic values. |
| • Uricosúricos | Decrease in the uricosauric effect and a decrease in the excretion of acetylsalicylic acid, resulting in higher plasma levels. |
| • Antiacids | They can increase the renal excretion of salicylates by alkalinization of the urine. |
| • I mean, | Increased plasma levels of digoxin that can reach toxic values. |
| • Barbiturates | It increases the plasma concentrations of barbiturates. |
| • Zidovudine | It increases the plasma concentrations of zidovudine by competitively inhibiting glucuronidation or directly inhibiting hepatic microsomal metabolism. |
| • Valproic acid | Decreasing the union to plasma proteins and inhibiting the metabolism of valproic acid. |
| • Fenitoin | Increased plasma levels of phenitoin. |
Finally, the following table shows non-drug interactions, manifested as alterations in laboratory test results:
| Analytical interferences of acetylsalicylic acid. | |
| Interaction results. | Affected analytical determinations. |
|---|---|
| Biological increase. | • alkaline phosphatase •transamines (ALT and AST). •amoniac •bilirubina •colesterol •creatin quinasa •creatinine •digoxine • free throxine •lactate dehydrogenase (LDH). •globulin connecting to thyroxine •triglycerides • uric acid Valproic acid |
| Biological reduction | • Estriol (orina). • free throxine •glucosa •pheny •TSH •TSH-RH •tiroxine •triglycerides •triyodothyronine • uric acid • creatinine clearing |
| Analytical increase | •glucosa •paracetamol •Total proteins |
| Analytical reduction | •transamines (ALT). •albumin • alkaline phosphatase •colesterol •creatin kinase •lactate dehydrogenase (LDH). •Total proteins • 5-hydroxylacetic acid (orina). • 4-hydroxy-3-metoxy-mandelic acid (orina). •total estrogens (orina). •glucosa (orina). |
| Note: The biological increase consists of a "true increase" of the determined value, as a result of interaction with the organism. The analytical increase is given by a "false elevation" of the determined value, as a result of the interaction with the reagents that form part of the technique that determines the levels of each one of them. Similarly, biological and analytical reductions occur. | |
In patients with established heart disease, ibuprofen may interfere with the cardioprotective effects of aspirin when both drugs are given at the same time.
Clinical use
- Mild and moderate pain of varied origin, such as headache, menstrual periods, colds, tooth pain and muscle aches.
However, it is not effective for severe visceral pain. Aspirin and other NSAIDs (non-steroidal anti-inflammatory drugs) have been combined with opioid analgesics for the treatment of cancer pain, where the anti-inflammatory effects work synergistically with opioids to increase analgesia. The combination of aspirin with oxycodone—a class of narcotic pain relievers—is used to relieve moderate to moderately severe pain.
- Kawasaki disease, especially at high doses during the fever phase.
- Rheumatic fever, especially rheumatoid arthritis.
- Autoimmune diseases type lupus erythematous.
- Other inflammatory disorders of the joints.
Analgesia or antipyresis
Doses to achieve the analgesic or antipyretic effects of aspirin are less than 0.6-0.65 grams (600-650 milligrams), taken orally. Higher doses can prolong the effect. The usual dose is usually repeated every 4 hours.
The anti-inflammatory dose in children is 50 to 75 mg per kg of the child's weight each day, divided into several doses throughout the day.
The average entry dose for an adult is 45 mg/kg/day in divided doses.
Cardiovascular protection
Aspirin decreases the incidence of ischemic attacks, unstable angina, coronary artery thrombosis with acute myocardial infarction, and thrombosis secondary to coronary bypass with a proposed daily dose (in 2020) of 75-162 mg.
Other uses
Certain epidemiological studies suggest that long-term use of low-dose aspirin is associated with a reduction in the incidence of colorectal cancer, as well as lung cancer, possibly because of its association with effects inhibitory on COX produced by adenocarcinomas, prostaglandin suppressive effects or even direct antimutagenic effects. The role that aspirin plays in reducing the incidence of other forms of cancer has also been studied. Several studies have shown that aspirin does not reduce the occurrence of prostate cancer. Its effects in preventing pancreatic cancer are mixed, with a 2004 study finding a statistically significant increased risk of pancreatic cancer in women taking aspirin, while a multi-study meta-analysis published in 2006 found no conclusive evidence for pancreatic cancer. that aspirin or other NSAIDs (non-steroidal anti-inflammatory drugs) were associated with an increased risk of this form of cancer. Aspirin may also have positive effects on cancer of the upper digestive tract, but the evidence remains inconclusive.
Aspirin has been hypothesized to be capable of reducing cataract formation in diabetic patients, although in at least one study it was shown to be ineffective in that action.
According to scientists at the Institute of Neuroscience and Physiology at the University of Goteborg in Sweden, a daily dose of aspirin can help reduce brain deterioration in the elderly. However, experts stress that "due to the dangerous side effects that 'Aspirin' can have, its massive use cannot be recommended to protect memory."
Adverse effects
At usual doses, the most common adverse effects of aspirin are gastric irritation, nausea, vomiting, gastric or duodenal ulcer, while hepatotoxicity, asthma, skin changes, and nephrotoxicity are less frequent. Mucosal adaptation has been documented to occur in patients with ulcers associated with aspirin administration such that spontaneous improvement over time without discontinuation of aspirin has been demonstrated.
Its abusive intake causes nephritis, which are inflammatory and/or dysfunctional processes of the kidney and peripheral vasodilatation by direct action on smooth muscle. At high doses, some patients have reported vomiting, tinnitus, decreased hearing, delirium, psychosis, stupor, and vertigo which are reversible by reducing the dose. At even higher doses of salicylates, profuse respiration and coma appear as a result of a direct effect on the medulla oblongata.
At toxic levels of salicylates, respiratory alkalosis occurs followed by metabolic acidosis due to salicylate accumulation, respiratory depression, cardiotoxicity, and glucose intolerance. Two grams or even less of aspirin a day can increase blood uric acid levels, while doses above 4 grams a day lower urates. Like all NSAIDs (non-steroidal anti-inflammatory drugs), aspirin can cause elevated liver enzymes, hepatitis, kidney dysfunction, bleeding, and asthma.
Bleeding
Some people are less affected than others by the antiplatelet effect of aspirin. One study has suggested that women are more resistant to the platelet effects of aspirin than men, and in another study, about 30% of patients tested were just as resistant to the secondary action of aspirin.
Slight GI bleeding can occasionally occur, usually caused by erosive gastritis, which over time can lead to iron deficiency. In its commercial form, it should not be administered to children under 12 years of age suffering from the flu or chickenpox (paracetamol is usually used instead) and/or used in conjunction with other salicylates, as it can lead to Reye's Syndrome, a disease rare, but very serious. The administration of aspirin during dengue fever is not recommended due to an increased risk of bleeding.
Administration of aspirin to mothers before delivery may cause hemostatic disturbances in newborns, including petechiae, hematuria, cephalohematoma, conjunctival hemorrhage, and bleeding during or after circumcision. For their part, mothers may present with bleeding confined to the intrapartum or postpartum period. Therefore, the administration of aspirin should be avoided during pregnancy and if it is suspected that the mother has taken aspirin in the five days before delivery, the newborn should be evaluated to rule out bleeding.
Regarding other adverse reactions (ADRs), and as a summary, a table is included following the CIOSM criteria.
| Adverse reactions to acetylsalicylic acid | ||
| System involved | CIOSM Group. | Type of reaction |
| Digestive device | Frequent | Gastric ulcer, duodenal ulcer, gastrointestinal bleeding (melenas, hematomesis), abdominal pain, dyspepsia, nausea, vomiting. |
| Few frequent | Hepatitis | |
| Respiratory device | Frequent | paroxysmal bronchial spasm, severe dyspnea, rhinitis. |
| Leather and subcutaneous tissue | Frequent | Urticaria, rashes, angioedema. |
| Few frequent | Sweating | |
| Blood and lymph system | Frequent | Hipoprotrombinemia |
| Nervous system | Few frequent | Dizziness, confusion, tinnitus, deafness |
| Genitourinary | Rare | Kidney failure and acute interstitial nephritis |
| Other | Few frequent | Reye syndrome, headache. |
| Rare | Anaphylactic or anaphylactoid reactions | |
Overall, it can be stated that between 5% and 7% of patients experience some type of adverse effect.
Overdose
Taking more than 150 mg/kg of aspirin can cause serious and even fatal results if left untreated. For a small adult, that's roughly equivalent to taking 20 tablets containing 325 mg of aspirin. Children may be affected with much lower levels.
Patients with an accidental or intentional overdose of aspirin undergo gastric lavage with activated charcoal, and profuse alkaline urine output ensues. If they present disorders such as hyperthermia or electrolyte imbalances, they must be restored. In severe poisoning, hemodialysis or, rarely, assisted ventilation may be necessary. Sodium bicarbonate infusions are often used to alkalize the urine leading to an increase in salicylate excreted out of the body.
Contraindications
The antiplatelet action of aspirin makes it contraindicated in patients with hemophilia. Although aspirin use during pregnancy was not recommended in the past, aspirin may be useful in the treatment of preeclampsia and even eclampsia.
Aspirin should not be given to individuals with a history of allergy to ibuprofen or naproxen, or who are otherwise intolerant to salicylates or NSAIDs (non-steroidal anti-inflammatory drugs), and moderation should be exercised in prescribing aspirin to patients with asthma or NSAID-induced bronchospasm. Due to its action on the stomach mucosa, it is recommended that patients with kidney disease, peptic ulcers, diabetes, gout or gastritis consult a health professional before taking aspirin. Even in the absence of these diseases, there is always a risk of gastrointestinal bleeding when aspirin is combined with liquor or warfarin.
Aspirin has been shown to cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, especially at large doses and depending on the severity of the disease.
Introductions
Regarding the doses, usually the lowest ones (300 mg or less) are usually used as an antiplatelet agent, since its use in children has been greatly restricted. The highest ones are used looking for the analgesic/antipyretic or antirheumatic effect. Regarding the presentations, it can be said that practically all the possible galenic forms for clinical use of acetylsalicylic acid have been studied. The ones described below are the most commonly used:
- 25 mg, 100 mg, 125 mg, 250 mg, 300 mg, 500 mg, 650 mg
- 500 mg chewable compounds
- 500 mg effervescents
- 100 mg and 300 mg coated compounds
- Efervescent granulate, 500 mg envelopes
- Capsules (associated to other drugs) for delayed presentation
- We can also find it in unusual dosages, such as 267 mg, associated with other substances.
Likewise, there are many presentations with other associated drugs, usually for the treatment of catarrhal processes. This is the case of associations with phenylephrine, chlorphenamine, caffeine, vitamin C (levorotatory ascorbic acid) or substances from the vitamin B complex. Other times it is associated with other drugs to increase its antiplatelet (dipyridamole) or analgesic (paracetamol) effect.
Regarding the usual excipients, a similar situation occurs. Both the pharmaceutical form and the organoleptic characteristics of the drug require the use of certain types of excipients. However, the large number of laboratories that manufacture it as a generic drug means that the excipients that we can find are highly variable. However, the ones that are usually included in the commercial form Aspirin (probably the most sold in the world) and some of the most common in the generic forms are listed below:
- Colorants:
- orange yellow S (E-110).
- yellow quinolein coloring (E-104).
- Conservatives and antioxidants:
- (E-200),
- Citrates: anhydric citric acid (E-330), sodium citrate (E-331).
- Stabilizers and emulgents:
- Powder cellulose (E-460 II).
- Calcium stearate (E-470 A).
- PH and anti-aglutinent regulators:
- Carbonates: Sodium carbonate (E-500), Sodium hydrogen carbonate (E-500 I), heavy magnesium carbonate (E-504).
- Others:
- Sweeteners, like aspartame (E-951), manitol (E-421) or corn starch (gelifying and thickening).
- Very variable aromatizers, including the aroma of mandarin juice, orange aroma or special dry aroma,
- Sodium, cellulose microcrystalline.
- The coated tablets usually carry in the outer layer copolymer of metacrylic acid type C, sodium dodecilsulfate (E-514), polysorbate 80, talc or triethyl citrate (E-1505).
Aspirin Substitutes
Because of the minor side effects of aspirin, and because some people are allergic to it, various substances can be used as substitutes. The most common is paracetamol, called acetaminophen in the US, which reduces fever and relieves pain much like aspirin, although it has no anti-inflammatory activity. Excessive use of acetaminophen can cause kidney and liver damage. A second common substitute is ibuprofen, which works like aspirin to reduce pain, fever, and inflammation. Another addition to the family of over-the-counter aspirin substitutes is naproxen. Its main advantage is its long-lasting effect. While almost all pain relievers require dosing every 4-6 hours, a single naproxen tablet lasts 8-12 hours.
Contenido relacionado
Oxytocin
Mustard
Otto Heinrich Warberg